“`html
When surgeons mend tissues, they are presently restricted to mechanical methods like stitches and fasteners, which can inflict additional harm, or meshes and adhesives that may not sufficiently bond with tissues and can be rejected by the organism.
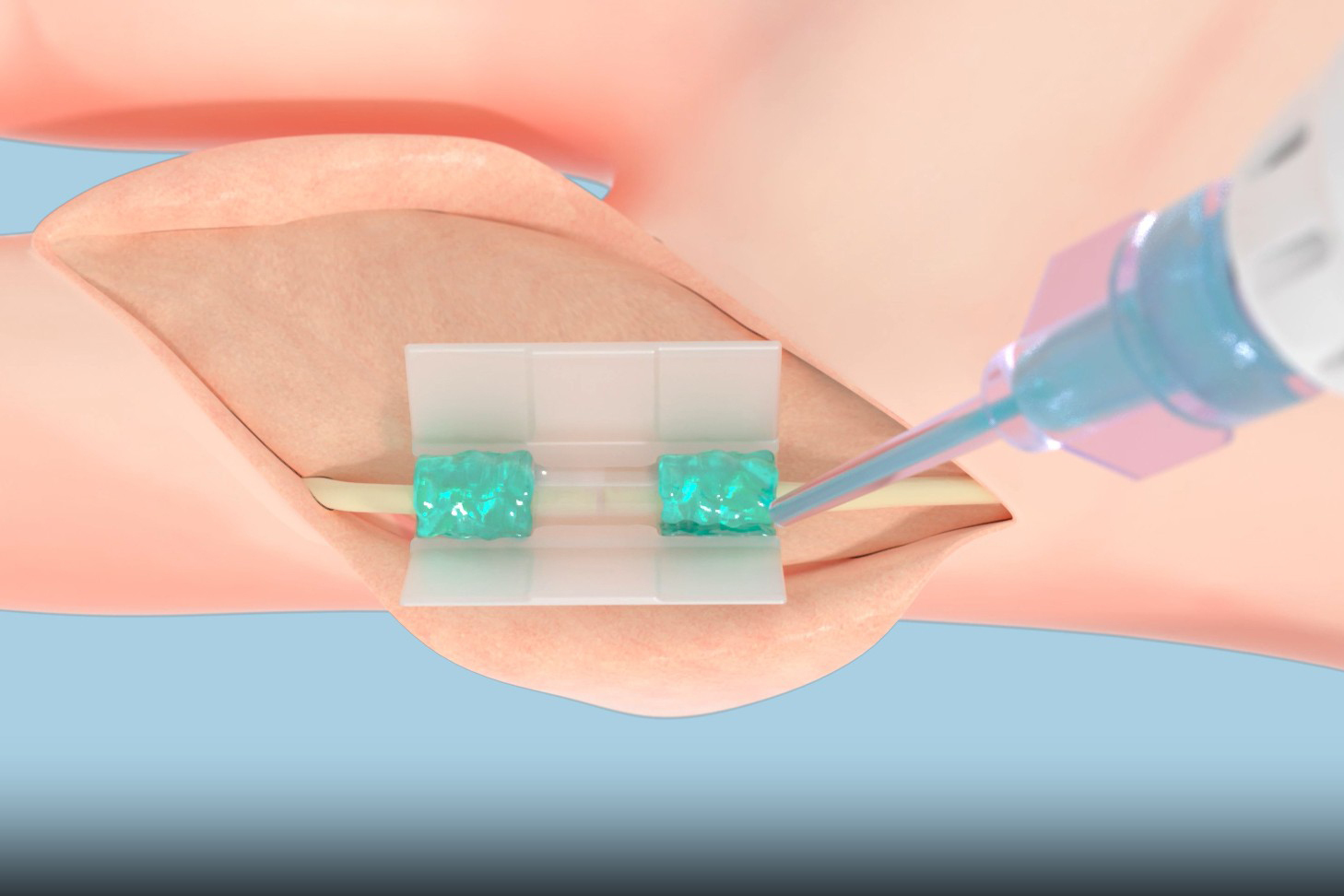
Now, Tissium is presenting a fresh solution for surgeons based on a biopolymer innovation originally developed at MIT. The firm’s adaptable, biocompatible polymers take the shape of surrounding tissues, adhering to them to mend damaged tissue once activated with blue light.
“Our aim is to establish this technology as the new benchmark in fixation,” states Tissium co-founder Maria Pereira, who began her journey with polymers as a PhD student through the MIT Portugal Program. “Surgeons have been utilizing stitches, fasteners, or tacks for decades or even centuries, and they can be quite invasive. We’re focusing on assisting surgeons in repairing tissues in a less invasive manner.”
In June, Tissium achieved a significant milestone when it obtained marketing authorization from the Food and Drug Administration for its non-invasive, suture-free method to repair peripheral nerves. The FDA’s De Novo marketing authorization acknowledges the uniqueness of the company’s platform and allows for the commercialization of the MIT spinout’s inaugural product. This followed studies indicating that the platform helped patients recover full flexion and extension of their injured fingers or toes without pain.
Tissium’s polymers can function across various tissue types, including nerves, cardiovascular structures, and abdominal walls, and the company is eager to apply its programmable platform to other areas.
“We genuinely believe this approval is merely the commencement,” asserts Tissium CEO Christophe Bancel. “It was a vital milestone, and it wasn’t straightforward, but we were aware that if we could secure the first one, it would transition the company into a new era. Now, we have the obligation to demonstrate its effectiveness in additional applications and how it can assist more patients.”
From lab to patients
Years prior to co-founding Tissium, Jeff Karp was a postdoc in the laboratory of MIT Institute Professor Robert Langer, where he focused on creating elastic materials that were biodegradable and photocurable for various clinical applications. Upon graduation, Karp took on an affiliate faculty role in the Harvard-MIT Program in Health Sciences and Technology. He is also a faculty member at Harvard Medical School and Brigham and Women’s Hospital. In 2008, Pereira joined Karp’s lab as a visiting PhD student funded by the MIT Portugal Program, fine-tuning the polymers’ thickness and water-repulsion properties to enhance the material’s capacity to adhere to moist tissue.
“Maria transformed this polymer platform into a fixation system applicable in numerous medical areas,” Karp recalls. “[The cardiac surgeon] Pedro del Nido at Boston Children’s Hospital informed us about a significant issue concerning a congenital defect resulting in holes in newborns’ hearts. There were no suitable remedies, so that became one of the applications we initiated work on, led by Maria.”
Pereira and her team went on to demonstrate that they could utilize biopolymers to seal heart holes in rats and pigs without bleeding or complications. Bancel, a veteran in the pharmaceutical industry, was introduced to the technology during a meeting with Karp, Pereira, and Langer while visiting Cambridge in 2012, and he spent subsequent months engaging with surgeons.
“I consulted with approximately 15 surgeons from various specialties regarding their challenges,” Bancel explains. “I recognized that if the technology could function within these settings, it would tackle numerous issues. All the surgeons were enthusiastic about the potential impact of the material on their practices.”
Bancel collaborated with MIT’s Technology Licensing Office to transition the biopolymer technology out of the lab, including patents from Karp’s initial work in Langer’s lab. Pereira relocated to Paris after earning her PhD, and Tissium was officially established in 2013 by Pereira, Bancel, Karp, Langer, and others.
“The MIT and Harvard ecosystems are fundamental to our success,” Pereira notes. “From the outset, we aimed to tackle problems that would significantly impact patients. We weren’t just conducting research for research’s sake. We commenced in the cardiovascular sector, but quickly recognized our aspiration to set new standards for tissue repair and fixation.”
After licensing the technology, Tissium had considerable work ahead to scale it commercially. The founders partnered with companies specializing in polymer synthesis and devised a method to 3D print casing for polymer-wrapped nerves.
“We soon discovered that the product comprises both the polymer and the accessories,” Bancel states. “It all revolved around how surgeons utilized the product. We had to create the appropriate accessories for specific procedures.”
The new system is ardently needed. A recent meta-analysis of nerve repairs using sutures revealed that only 54 percent of patients achieved significant recovery post-surgery. By avoiding sutures, Tissium’s adaptable polymer technology offers a less invasive method for reconnecting nerves. In a recent trial with 12 patients, all who completed follow-up regained full flexion and extension of their injured digits, reporting no pain 12 months after the procedure.
“The current standard of care is far from optimal,” Pereira remarks. “There are fluctuations in outcomes, sutures may inflict trauma, tension, misalignment, and all these factors can influence patient results, from sensation to motor function and overall quality of life.”
Trauma-free tissue repair
Currently, Tissium has six products in development, including one ongoing clinical trial related to hernias and another about to commence for a cardiovascular application.
“Early on, we had the intuition that if this technology was effective in a single application, it would be surprising if it didn’t succeed in multiple other areas,” Bancel reflects.
The company also believes its 3D-printed production method will facilitate expansion.
“This can not only be utilized for tissue fixation broadly across medicine but we can also leverage the 3D printing process to manufacture all sorts of implantable medical devices from the same polymeric platform,” Karp elucidates. “Our polymers can be programmed, allowing us to tailor the degradation, the mechanical properties, paving the way for other exciting advances in medical devices with innovative capabilities.”
Now the Tissium team is urging individuals within the medical community to contact them if they think their platform could enhance the standard of care — and they’re aware that the initial approval is a commendable milestone in its own right.
“It’s the ideal scenario for your research to yield not just a publication, but a treatment that has the potential to elevate the standard of care and improve patients’ lives,” Karp expresses. “It’s the dream, and it’s a remarkable feeling to celebrate this achievement along with all the collaborators who have been part of the journey.”
Langer adds, “I concur with Jeff. It’s fantastic to witness the research we initiated at MIT reach the stage of FDA approval and transform lives.”
“`