“`html
Health
Realizing the potential of CAR-T
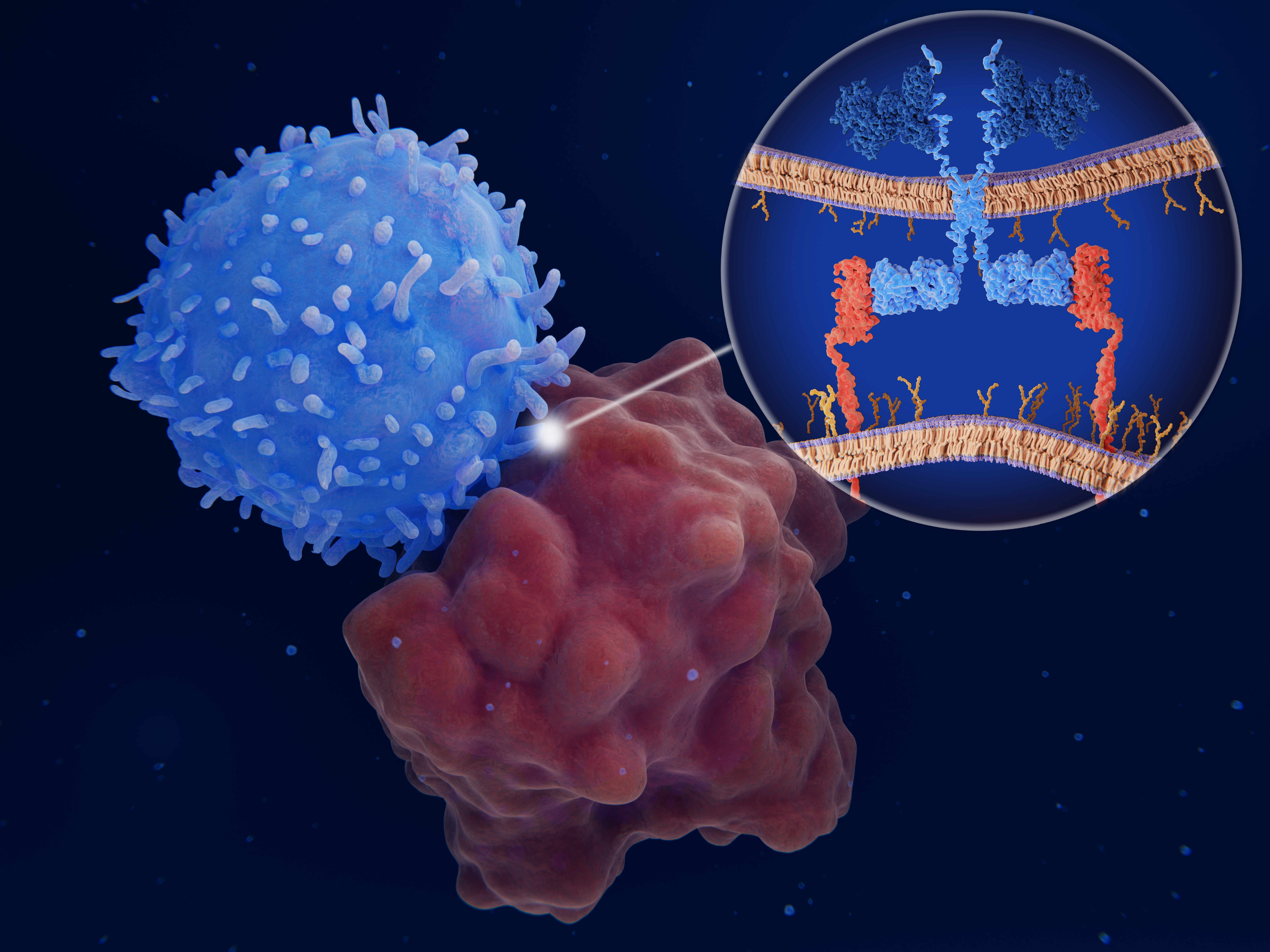
Investigations on multiple fronts aim to enhance the influence of a cancer treatment that has astounded both patients and medical professionals
David Avigan is hesitant to describe CAR-T-cell therapy as a “miracle,” yet he comprehends what it signifies to those who choose to use the term. He recalls the initial instance it occurred with one of his patients.
“As a discipline, we tend to be somewhat reserved — our patients experience ups and downs,” noted Avigan, who leads the Cancer Center at Beth Israel Deaconess and holds the Theodore W. and Evelyn G. Berenson Professorship of Medicine for Oncology Studies at Harvard Medical School. “Yet, to be honest, we have witnessed exceptionally remarkable reactions in patients with advanced illnesses.”
Initially sanctioned by the FDA in 2017, CAR-T-cell therapy mobilizes the immune response against cancer. It has sparked swift recovery in some of the most critically ill patients, whose hopes had diminished after several unsuccessful treatments. Doctors report incredible outcomes: tumors vanishing in mere weeks or even days, and individuals who were close to death rising up and resuming their normal routines.
“They had undergone every available therapy, whether known or experimental — nothing was effective — and post-CAR-T therapy, they went into remission, and just a week later, were moving about as if they were entirely healthy, even if they had suffered significantly during the treatment,” shared Robbie Majzner, an associate professor of pediatrics at the Medical School and head of the Pediatric and Young Adult Cancer Cell Therapy Program at the Dana-Farber/Boston Children’s Cancer and Blood Disorders Center. “We had one patient who nearly died during the treatment, yet two weeks later he was free of leukemia and snowboarding. It was simply astonishing.”
Eric Smith, an assistant professor of medicine at the Medical School and the director of translational research for immune effector cell therapies at Dana-Farber, describes CAR-T as a “platform” rather than a singular therapy. Its primary asset is one of the body’s strongest defenders — the T-cell, honed over eons to eliminate bacteria, viruses, fungi, and other intruders. The CAR, or chimeric antigen receptor, serves as the T-cell’s targeting mechanism, which can be fine-tuned by bioengineers to hit various targets. This adaptability enabled Smith and colleagues to evolve a treatment initially effective against leukemia and lymphoma into a solution for another blood malignancy, multiple myeloma. It also fuels enthusiasm surrounding the therapy’s capability to contend with not only other cancers but also non-cancerous disorders like autoimmune diseases and persistent infections.
“The platform is exceptionally versatile for further enhancements — the various approaches we can employ to boost efficacy — that we’re quite optimistic,” Smith remarked. “We’re poised to cure a greater proportion of patients with the next iteration.”
Eric Smith noted he has witnessed multiple extraordinary recoveries. One of his initial cases was the second patient ever to receive CD-19 directed CAR-T-cell therapy while in his oncology fellowship at the Memorial Sloan Kettering Cancer Center in New York City.
“We were astounded to witness such an incredible clinical reaction,” Smith mentioned. “We published a case report on a patient who had rapidly advancing myeloma that, between initiating conditioning therapy and acquiring her CAR-T-cells, led to paralysis due to the myeloma in her spine. Subsequently, with the CAR-T-cells, she managed to recover from that and thrive for over a year. So indeed, this is one aspect that distinguishes it significantly from other treatments.”
Veasey Conway/Harvard Staff Photographer

Prior to the advent of CAR-T and various immunotherapies, cancer cells were shielded from immune assault because they originate from the patient’s own cells and were not recognized as intruders by the immune system.
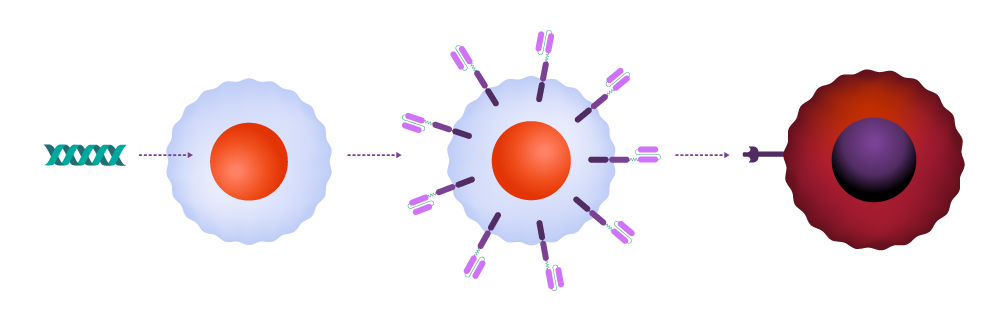
In CAR-T therapy, clinicians extract T-cells from the patient and transport them to a cell manufacturing facility, where the CAR is incorporated into the cell membrane. The modified cells are then multiplied, frozen, and returned to the medical facility for infusion back into the patient. Once inside the body, the CAR binds to a molecule — the antigen — on the cancer cell surface, signaling the T-cell to launch an attack.
How it functions
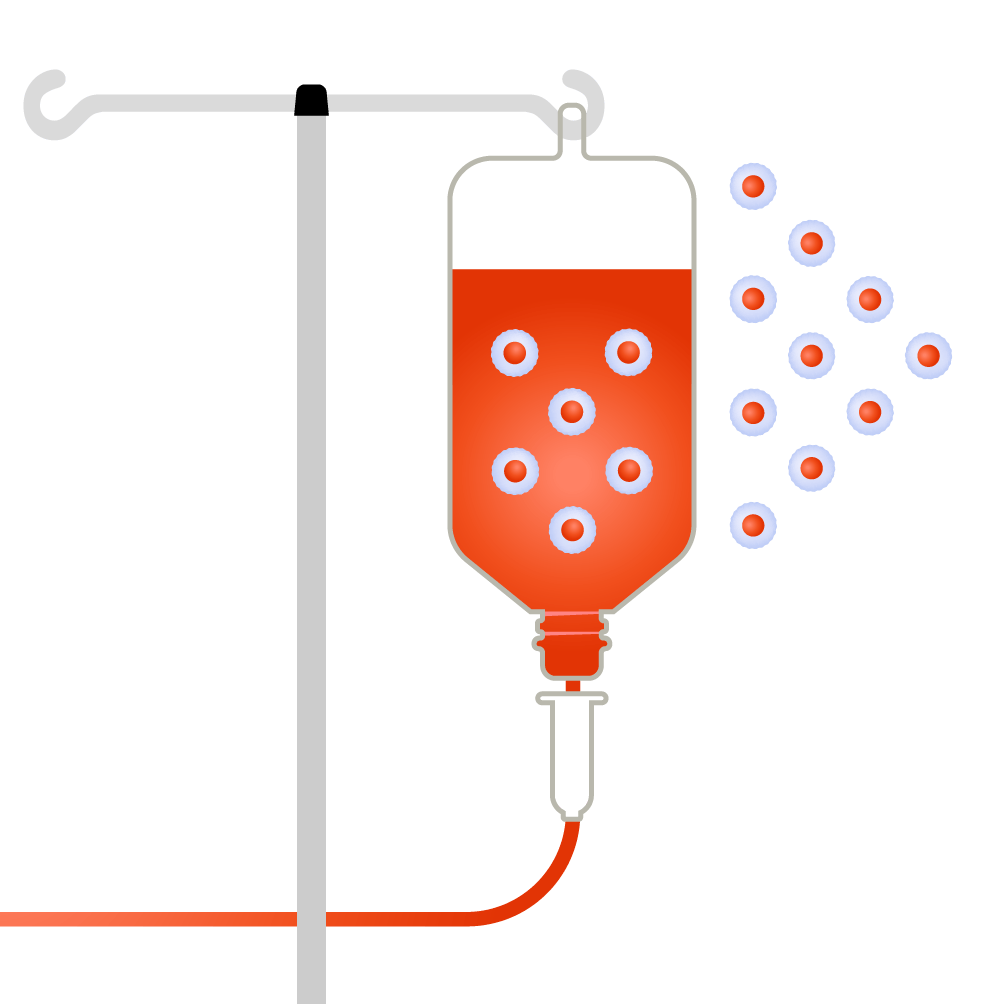
T-cells are harvested from a patient.
Source: Leukemia and Lymphoma Society; illustrations by Judy Blomquist/Harvard Staff
“““html
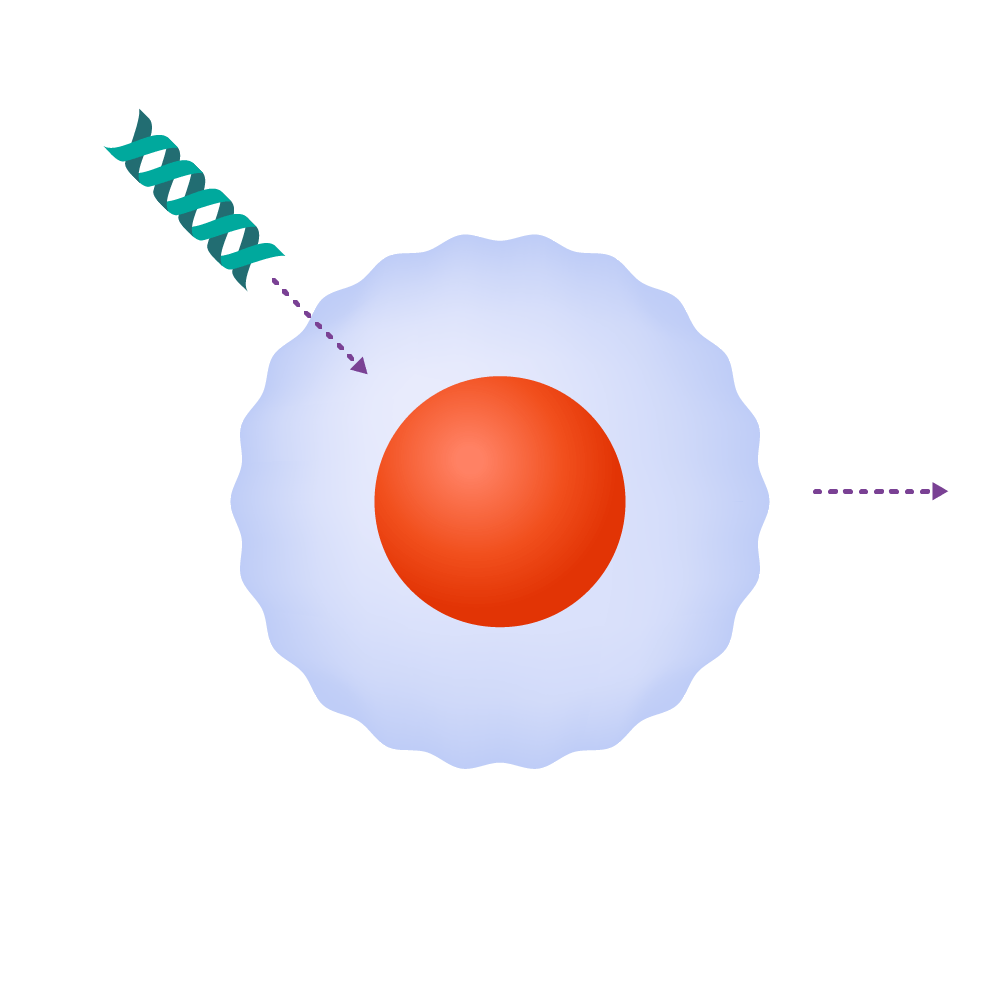
T-cells are modified genetically to produce chimeric antigen receptors, often referred to as CARs.
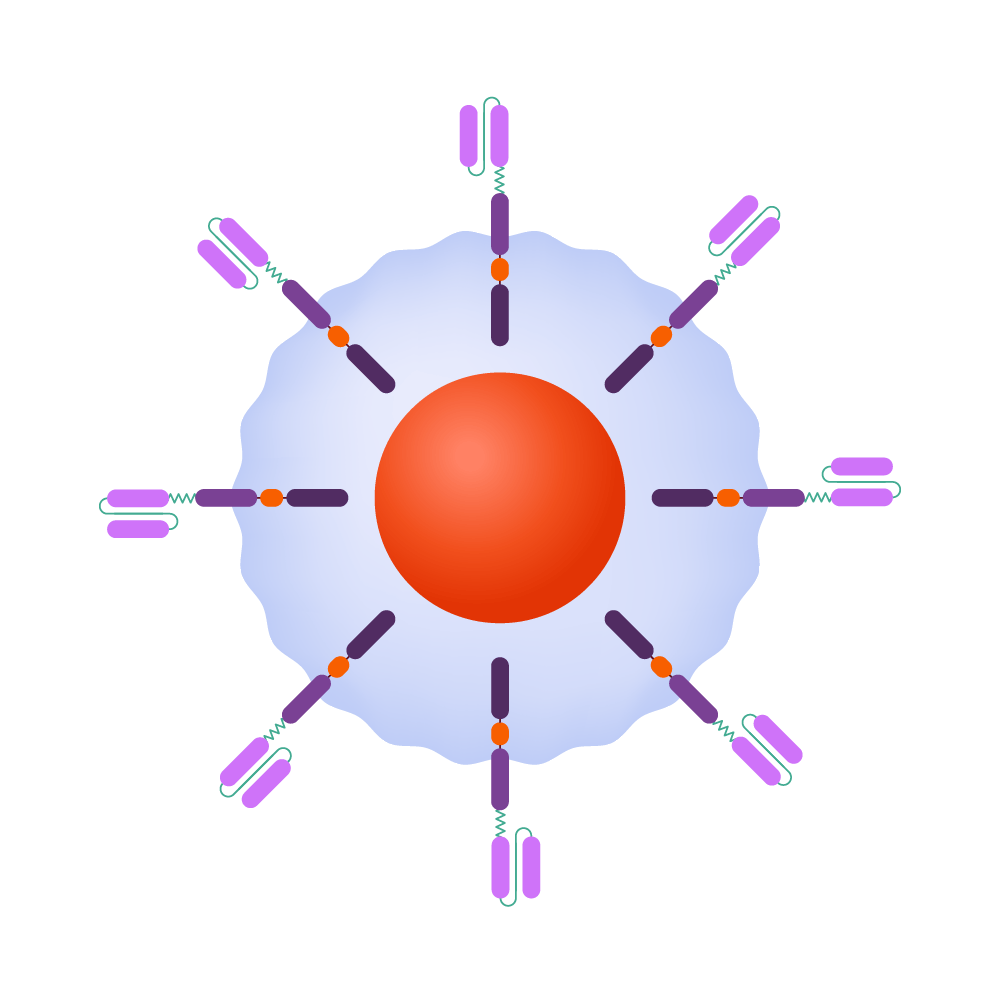
CAR-T-cells can identify and eliminate malignant cells, while also providing protection against recurrence.
The modified cells are reproduced until millions of these attacking cells are present.
CAR-T-cells are reintroduced back into the patient.

Within the body, the CAR binds to an antigen found on a cancer cell’s surface.
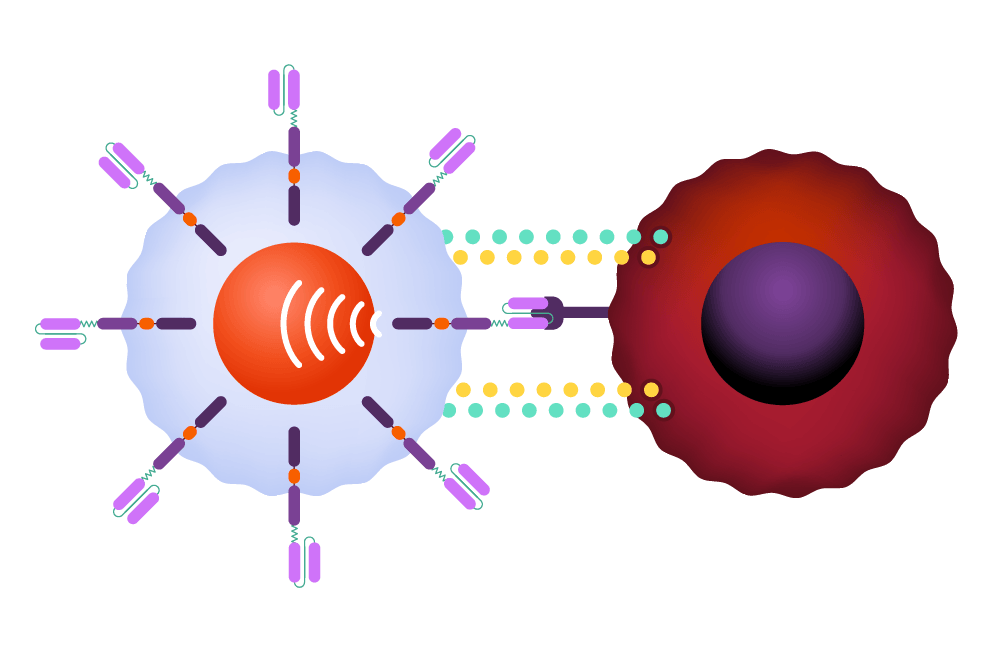
The CAR instructs the T-cell to release potent cytotoxins, leading to cell destruction.
The treatment has shown the greatest efficacy against leukemia, lymphoma, and myeloma, which collectively accounted for 9 percent of cancer cases in the U.S. last year, impacting 187,000 individuals. Experts assert that expanding these advances to solid tumors represents a crucial new frontier, illustrating the gradual yet steady progress typical of scientific advancement.
Among the significant challenges, along with the issues posed by solid tumors, is the fact that CAR-T is not universally effective. Current remission rates range between 50 and 90 percent, contingent on the specific condition, and cancer remains the leading cause of death for those receiving treatment.
“I wish I could claim that all cell therapies are curative for everyone,” remarked Marcela Maus, a professor at the Medical School and director of the Cellular Immunotherapy Program at Mass General. “We haven’t arrived at that point, but there is potential and promise for some patients. Cells are living entities with their own ‘thermostat’ to regulate growth or shrinkage based on environmental signals. This opens doors to possibilities in regenerative medicine or single-shot cures that are challenging to achieve with other methods.”

Marcela Maus.
Kate Flock/Massachusetts General Hospital
One of Maus’ main focuses is addressing the solid tumor issue, which arises partly due to cellular diversity, the compactness of the mass, and threats to CAR-T cell survival within the aggressive immune environment surrounding the tumor. Last year, she collaborated with Harvard neurosurgeon Bryan Choi to evaluate the treatment against glioblastoma, a severe brain tumor that results in over 90 percent of patients dying within five years. They addressed the diversity challenge by designing a dual-target CAR that aimed at two molecules generally present on different cancer cells, thereby enhancing the T-cells’ assault. The first three participants in the study exhibited remarkable improvements in their cancer condition, although only one saw sustained effects. Follow-up findings on a total of 10 patients were presented at the American Society of Clinical Oncology this month.
Other research teams have reported varying outcomes in trials targeting lung, prostate, ovarian, and gastric tumors.
“There’s been significant hope for the technology to dramatically enhance the lives of patients with other diseases for quite some time,” stated Maus. “That aspect is still somewhat nascent and has been less straightforward to accomplish.” However, she has witnessed enough to feel optimistic about the “considerable promise” for breakthroughs that strengthen the fight against a broader spectrum of cancers.
Another major concern with CAR-T therapy relates to the side effects, which can be severe — a 2024 study attributed side effects to nearly 12 percent of non-cancer-related deaths among CAR-T patients — prompting some physicians to hesitate in recommending this treatment. One of the body’s responses to the therapy is an overreaction that releases proteins called cytokines, amplifying the immune signal. Cytokine release syndrome can be intense, with symptoms such as nausea, vomiting, rapid heart rate, and hallucinations. Another serious side effect is neurotoxicity syndrome, in which an exaggerated immune response impacts the brain, leading to temporary confusion in mild instances and coma in severe cases.
For patients who have exhausted all other alternatives, CAR-T therapy, despite its side effects, is nearly always deemed worthwhile, according to Avigan. For those not as advanced in treatment, the decision becomes more complex, but several studies indicate that CAR-T interventions provide benefits over standard therapies, he noted. One…
“““html
A significant rationale for administering CAR-T earlier is that multiple cycles of chemotherapy severely impact the body, reducing the efficacy of T-cells in patients with advanced stages of cancer. Transitioning CAR-T from a last option to a secondary treatment could harness T-cells that are more vigorous and proficient at eliminating cancer from the system.

David Avigan, who has cared for numerous CAR-T-cell patients since the therapy was introduced in 2017:
“There’s no doubt that for those of us engaged in this field, the transformational essence of this makes it an extraordinary honor to support patients and assist them in ways we couldn’t previously. I’ve managed many patients with CARs and have observed all facets of that range.”
Stephanie Mitchell/Harvard Staff Photographer
“It’s not that this is an ideal treatment for everyone regardless of their circumstances,” Avigan noted. “But yes, one can step back and say, ‘Wow, we are in a better position than we were a decade ago.’”
Foundational elements of a breakthrough
1987: Yoshikazu Kurosawa first articulates the concept of engineered CAR-T-cells capable of targeting specific cancer cells.
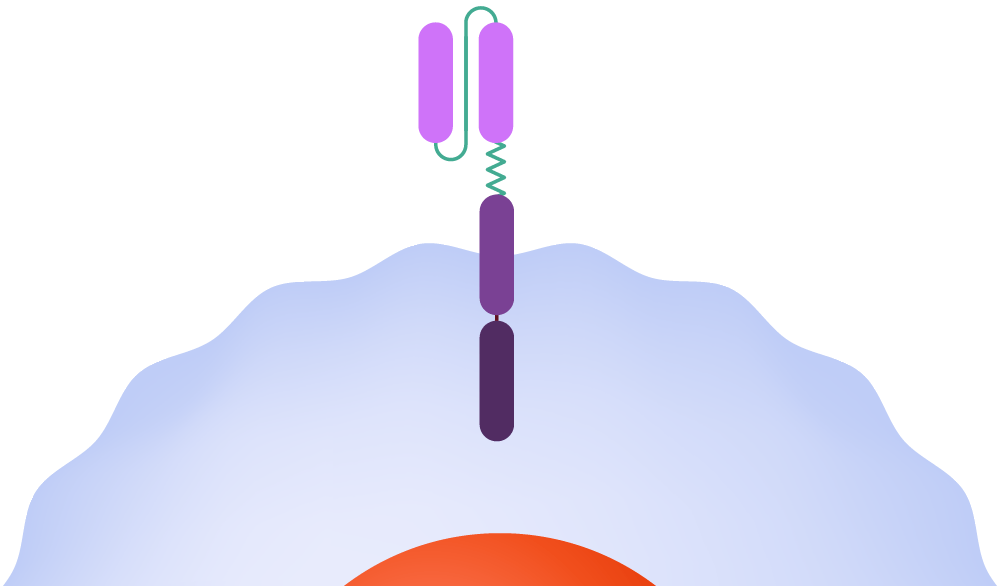
1989-1993: First-generation CAR-T-cell therapies are created by Zelig Eshhar and Gideon Gross, but the engineered cells fail to thrive in the body and are ineffective in clinical trials.
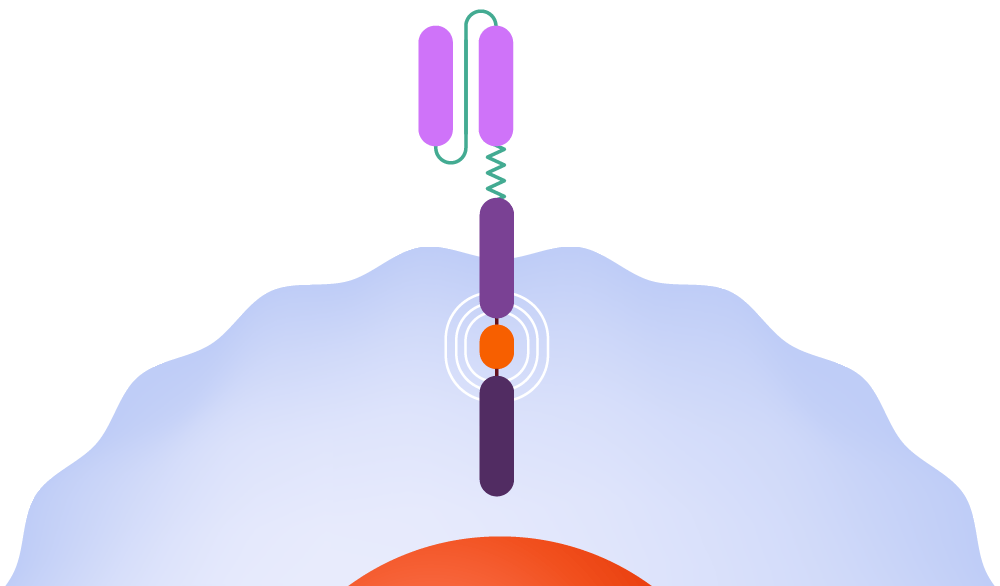
1998-2003: Michel Sadelain integrates a co-stimulatory molecule into CAR-T-cells, empowering them to remain active in the body. The team also adapts CAR-T-cells to focus on a protein present on the surface of cancerous cells in conditions such as leukemia and lymphoma.
2011: Three adult patients with advanced chronic lymphocytic leukemia experience full or partial remission following treatment with a tailored CAR-T-cell therapy.

2012: Six-year-old Emily Whitehead becomes the inaugural pediatric patient to undergo CAR-T-cell treatment. She recovered and continues to thrive. AP photo

2017: The initial CAR-T-cell treatments receive approval from the FDA. Numerous others gain approval in the subsequent years.
2024: By integrating two methods, CAR-T and bispecific antibodies known as T-cell engaging antibody molecules, a Mass General Brigham team witnesses significant regression in three glioblastoma patients. This research is especially crucial, as tumors can be more complex to address compared to blood malignancies.
2025: Research indicates that one-third of 97 individuals with multiple myeloma, previously deemed untreatable, remain alive and show no progression five years post CAR-T-cell treatment.
In the decade to come, CAR-T researchers anticipate overcoming or eliminating obstacles to the treatment’s efficacy as a cancer combatant — including affordability — while also evaluating its capabilities against noncancerous ailments.
Mohammad Rashidian, a scientist and faculty member at Dana-Farber, has developed an “enhancer protein” aimed at tackling two of CAR-T’s limitations: T-cell fatigue that results in a diminished initial response and waning responses over time, as observed in myeloma cases.
The enhancer protein, detailed in a study released last year, connects the CAR to an immune system signaling molecule named IL-2. This molecule boosts T-cell action and assists in nurturing memory CAR-T-cells, which could offer defense against cancer similarly to how infections or vaccinations protect against diseases.

Mohammad Rashidian.
Niles Singer/Harvard Staff Photographer
“I’m very hopeful,” Rashidian stated. “The data available is genuinely surpassing our expectations. Enhanced T-cells translate to significantly improved tumor eradication. If replicated in patients, we would expect markedly better results, and ideally, many patients could be cured.”
CAR-T’s prospects also extend to autoimmune disorders such as lupus, whereby the immune system mistakenly attacks the body. For this version of the treatment, Maus explained, the CAR is tailored to guide the CAR-T cell towards B-cells, which are responsible for the autoimmune assault in lupus. The therapy appears to reset the immune system, she added, reducing the B-cell assault in the months following treatment.
The reason for that reset remains unclear, Maus noted, but researchers are becoming increasingly intrigued by employing this therapy for other autoimmune disorders, including Type 1 diabetes. “There’s a significant array of autoimmune conditions that could potentially see substantial therapeutic advantages,” she emphasized.
The advantages of CAR-T come with a steep cost, with a single cancer therapy exceeding $400,000. Researchers remain optimistic that advancements in care and drug design, among other improvements, will serve as a counterbalance. Smith from Dana-Farber is particularly intrigued by the potential to eliminate the cell manufacturing facility from the production process.
A crucial phase in the bioengineering process is the vector’s delivery of the CAR’s gene to the T-cell. The cell utilizes these instructions to construct the CAR on its surface before it’s reintroduced into the patient. Currently, that step occurs in a facility, but Smith and colleagues are exploring a method that would directly inject the vector carrying the CAR gene into the patient. This vector would impart the genetic directives for the CAR to the T-cell while still inside the body, stimulating the T-cell to generate the corresponding CAR on the cell surface without needing a stop at a facility.
This concept still confronts considerable challenges, including potential off-target impacts if the vector is delivered to cells other than T-cells, but Smith claims it could revolutionize the process, simplifying a cumbersome experience for patients and reducing expenses.
Majzner from Boston Children’s is navigating a different landscape than that of adult cancers, partly because pharmaceutical companies prefer not to restrict themselves to treatments that only cater to pediatric cases, which are considerably fewer. His solution is a CAR that targets a molecule present on cells in various cancer types. Named B7-H3, it would enable drug manufacturers to develop CAR-T cells for pediatric patients with an array of cancers, expanding the patient demographic and potentially igniting more interest in developing therapies for young patients.

Robbie Majzner, reflecting on how witnessing CAR-T’s effects firsthand influenced his trajectory:
“Previously, I always viewed lab research as rocket science, filled with pathways I didn’t wish to memorize and far removed from patients. However, after working in Building 10 at the NIH, where the therapy had been directly developed and applied to patients, I recognized how close those processes actually are. This realization motivated me to engage in research. It surely wouldn’t have unfolded that way had I not observed such responses.”
Niles Singer/Harvard Staff Photographer
“We’ll initiate trials for that in solid tumors as well as brain tumors,” Majzner mentioned. “Perhaps it will advance for patients currently receiving treatments for pediatric solid tumors that appear unchanged from the past 40 years. Success would undoubtedly transform the approach we take to these cancers.”
Furthermore, it would further motivate specialists who have repeatedly witnessed the capabilities of CAR-T over the years. As Avigan remarked: “For those of us entrenched in this field, the transformative nature of this work makes it an incredible privilege to care for patients and assist them in ways we couldn’t have before.”
“`
