“`html
Envision a living cell as if it were a metropolis. If you were the urban designer for this (tiny, vibrant) metropolis, one of the decisions you’d need to make is how to distribute space among various functional roles.
Some areas of the metropolis should be designated for residential purposes, for instance, while others should be reserved for industrial use; simultaneously, you need to have a place for waste. Ideally, all these vital operations should be segregated so they don’t interfere with one another.

As a city expands, its various compartments must also enlarge. This holds true for a living cell as well. But do all components grow at the same pace? How do you allocate resources for growth?
Until recently, researchers have had limited insight into how the cell organizes and prioritizes the expansion of its compartments: the functional structures referred to as organelles. This encompasses several of the more recognizable organelles, like the cell’s nucleus or its energy-producing mitochondria.
A recent study directed by Shankar Mukherji, an assistant professor of physics in Arts & Sciences at Washington University in St. Louis, is among the first to adopt a systems approach to measuring and interpreting the transformations that occur in organelles as living cells mature. His research team published their findings on June 6 in Cell Systems, utilizing rainbow yeast cells for their study system.
“We employed a technique known as hyperspectral imaging, which enables us to label nearly all of the significant organelles within the cell, allowing us to directly observe how the cell assigns space to these compartments,” Mukherji stated.
Although his team did not create hyperspectral imaging, Mukherji noted they are among the initial few researchers to implement it for comprehending cell growth.
This innovative methodology permitted them to simultaneously visualize six key metabolically active organelles. Instead of examining singular or pairwise correlations among the organelles, as had been typical before, the physicists could structure their experiments and let the data reveal what was happening with all the organelles collectively.
“Using methods from data science, we were able to observe how the cells organized themselves in terms of the space allocated for organelles as we manipulated the conditions they were cultivated in or altered their signaling pathways,” Mukherji explained.
Mukherji hopes that the techniques they developed — along with the new mathematical theoretical framework his group constructed during this analysis — will aid others exploring how cells manage metabolism and growth, which is crucial in both health and illness.
Creating space
The researchers uncovered that certain organelles within a cell expand more rapidly than others.
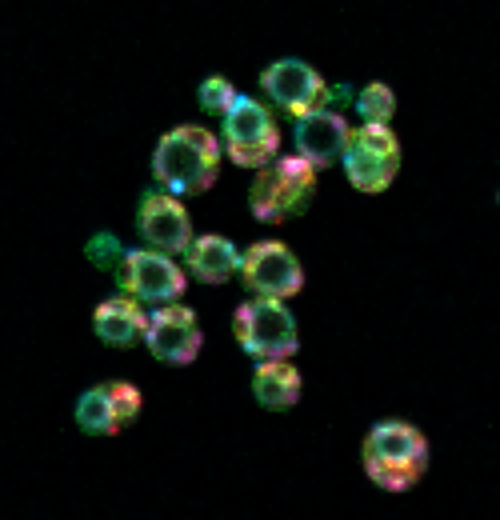
“The cell must execute something clever to allow compartments to expand, but not uniformly,” Mukherji remarked. “Fundamentally, it seems to prioritize certain organelles over others to meet elevated metabolic demands.”
They also noted that one specific organelle, the vacuole, appears to play a substantial role in assisting the cell to grow at a steady pace within a stable environment.
“The vacuole seems to be particularly proficient at buffering the cell against unpredictability,” Mukherji stated. “Simultaneously, if the cell genuinely needs to adjust its growth rate — due to environmental changes or other factors — then this is the organelle that appears to respond appropriately.”
Ultimately, the researchers examined what their data indicated about what triggers changes in organelle growth.
“With all the challenges we presented to the cells, it turns out that the pattern of organelle growth triggered by alterations in cell size is entirely distinct from the pattern instigated by changes in growth rate alone,” Mukherji explained.
This is significant because it may help clarify why eukaryotic cells exhibit such flexibility regarding how their sizes and the rates at which they grow and metabolize are interconnected.
“If conflicting demands from size and growth rate can independently communicate with the entire cell, perhaps it simplifies the process for the overall system to balance these competing requirements,” he stated.
As a subsequent step, Mukherji and his team intend to apply these same concepts to characterize human cells. “It’s likely that we’ll discover that the typical relationship between a cell’s organelle composition, its size, and its growth and metabolic attributes becomes altered in conditions that involve abnormal metabolism, ranging from cancer to diabetes to immune disorders,” he noted. “Whether we can uncover these patterns — and whether they are solely diagnostic, prognostic, or fundamentally related to disease — is something we’re eager to start exploring!”
The post Uncovering how cells allocate space to make way for new growth appeared first on The Source.
“`

