Health
The brain’s guardians
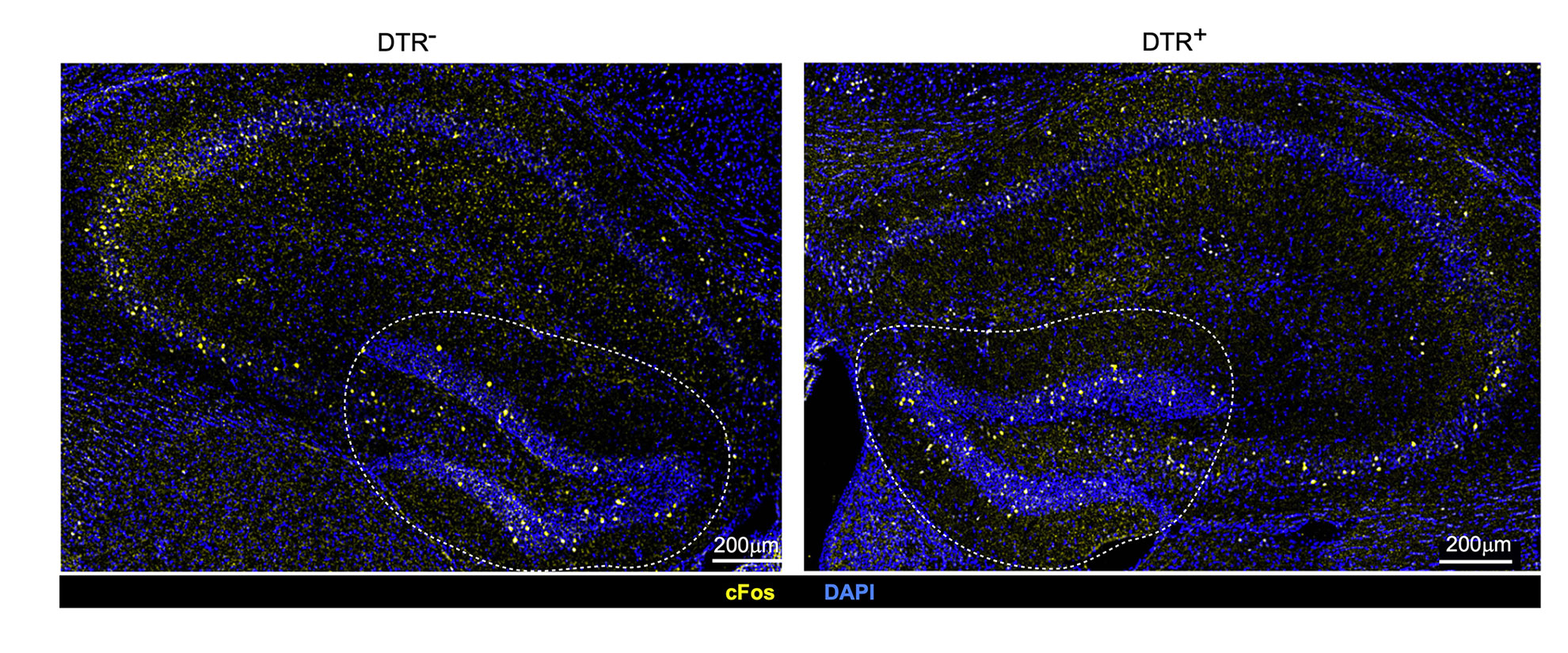
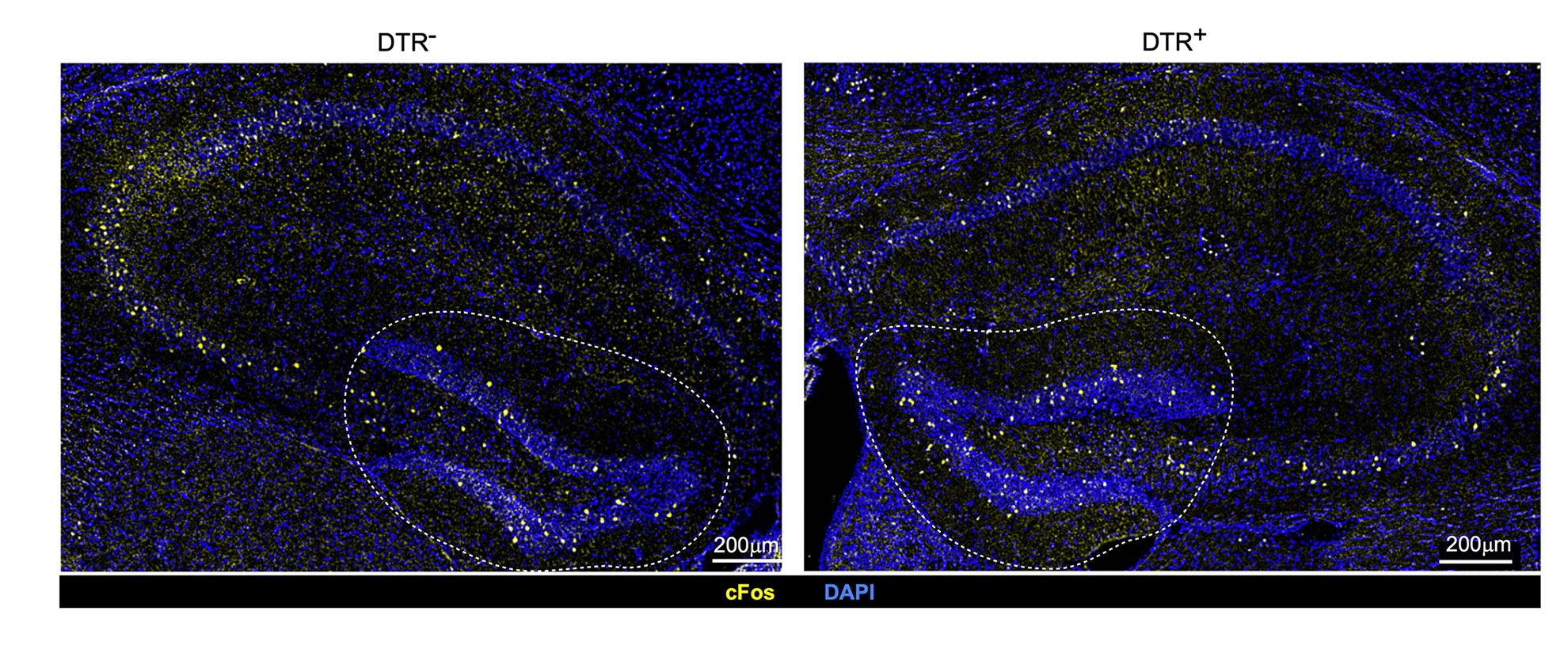
Variances in neuronal stimulation in mice with intact immune cells known as regulatory T cells or Tregs (left) and depleted Tregs (right). The discovery illustrates that Tregs contribute to maintaining stable neuronal activity under typical circumstances.
Credit: Mathis/Benoist Lab
HMS research identifies a unique class of cells that protect immunity and memory, potentially paving the way for treating neurodegenerative diseases
Regulatory T cells, known for their function in reducing inflammation, have been recognized for some time. In the context of infections, these Tregs prevent the immune system from becoming overly reactive and mistakenly attacking the body’s own tissues.
Recently, researchers at Harvard Medical School have identified a unique subset of Tregs residing within the protective membranes of healthy mouse brains, with their functions extending far beyond just controlling inflammation.
The study, released this Tuesday in Science Immunology, reveals that these specialized Tregs not only regulate access to the deeper areas of the brain but also facilitate the appropriate regeneration of nerve cells in a region associated with the formation and retention of short-term memories.
This investigation, partially supported by the National Institutes of Health, signifies a significant advancement in unraveling the intricate interactions of immune cells within the brain. If these results are further validated in subsequent animal experiments and affirmed in humans, they could lead to novel strategies for preventing or alleviating inflammation-related diseases in the brain.
“We uncovered a previously uncharacterized, distinct compartment of regulatory T cells located in the meninges surrounding the brain that performs a variety of protective roles, serving as gatekeepers for other immune cells and contributing to nerve cell regeneration,” stated study senior author Diane Mathis, the Morton Grove-Rasmussen Professor of Immunohematology at HMS’s Blavatnik Institute.
This research adds to a growing collection of findings that indicate Tregs fulfill roles beyond their conventional immune-regulatory responsibilities, acting as specific guardians of health, as noted by the researchers. Previous studies led by Mathis demonstrated that Tregs found in muscles activate during strenuous exercise to combat inflammation induced by activity while preserving muscle health.
“The Tregs discovered in the meninges possess abilities tailored to meet the requirements of this specific tissue,” remarked study lead author Miguel Marin-Rodero, a doctoral student within the immunology program at Harvard Medical School in the Benoist-Mathis lab. “These findings align with other research indicating that Tregs activate and deactivate specific genes to align with the identity and necessities of the organ they inhabit — they truly are the epitome of immune cells.”
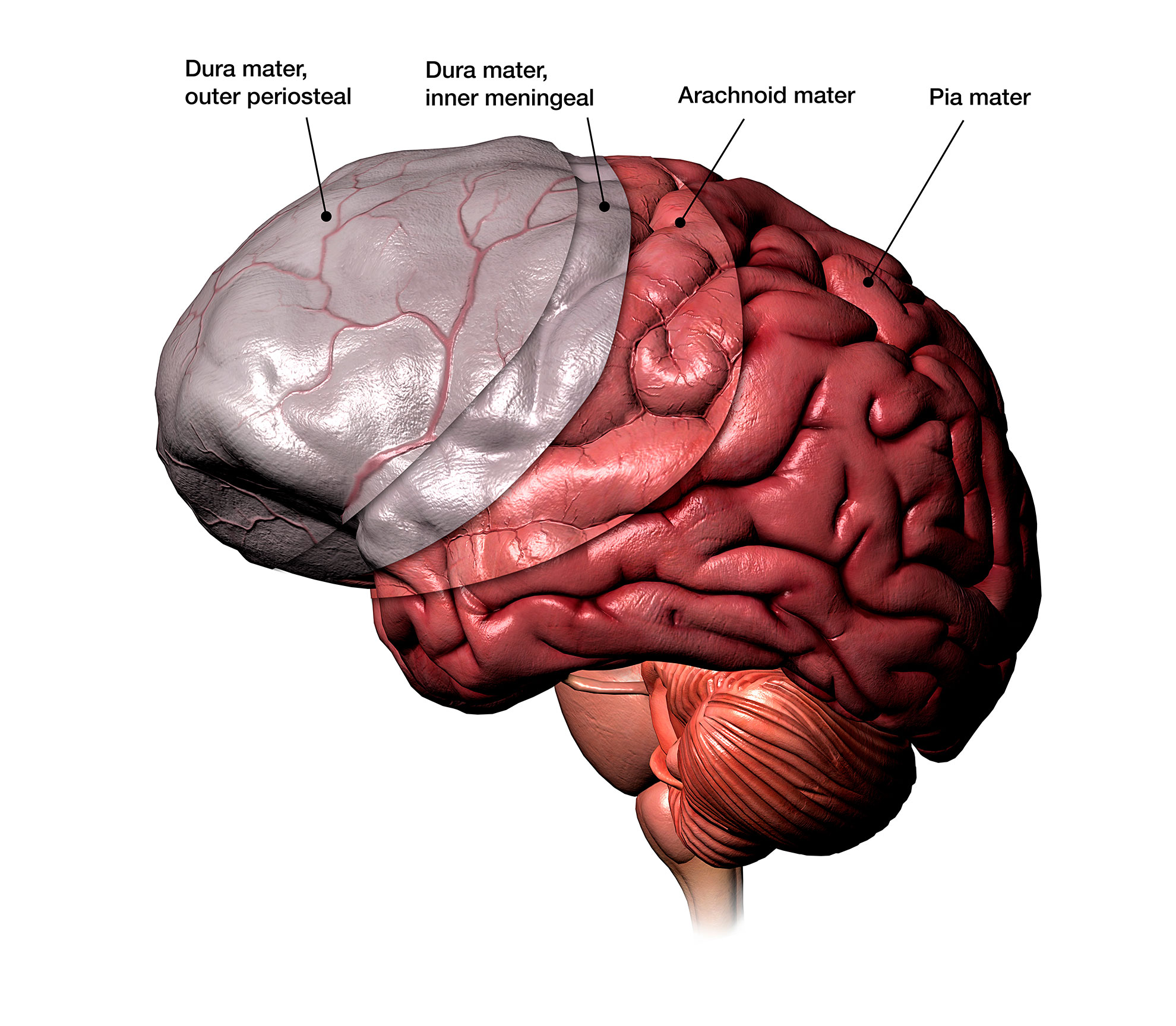
Illustration depicting the three protective layers beneath the skull.
Hank Grebe, 2018/Getty Images
Tregs residing at the brain boundary serve as gatekeepers
The meninges, consisting of three protective tissue layers nestled beneath the skull, safeguard the brain and spinal cord from harm, toxins, and infections. This border of the brain contains a varied assembly of immune cells. While the majority of these cells are innate and their functions have been relatively well understood, the adaptive immune cells—many of which emerge postnatally—have had their specific roles in brain immunity remaining somewhat enigmatic. The new research details the characteristics of Tregs—a specific type of adaptive immune cell—at the body-brain interface.
To clarify the function of Tregs in this scenario, the researchers implemented a genetic method to eliminate them from the meninges of mice. The meninges of animals deficient in Tregs exhibited elevated-than-normal levels of the inflammatory substance known as interferon-gamma, leading to extensive inflammation of the meninges. The absence of Tregs also exposed the brain’s interior areas to interferon-secreting, inflammation-promoting immune cells, triggering additional immune cells located nearby that are typically inhibited by Tregs to enter the brain and inflict widespread inflammation and tissue injury. The induced inflammation, the researchers observed, mirrored the damage and immune activity noted in human and mouse brains affected by Alzheimer’s disease.
“These experiments illustrate that Tregs in the meninges play a pivotal role as gatekeepers to protect the innermost parts of the brain,” told Marin-Rodero.
Loss of Tregs results in damage to a memory-processing area of the brain
Subsequently, researchers analyzed how the depletion of Tregs impacted distinct regions of the brain. Not all regions were equally influenced. In the absence of Tregs, inflammatory cells predominantly congregated in the hippocampus, a brain region integral to learning, memory acquisition and retention, as well as spatial orientation. The hippocampus stands out as one of the few areas in both rodent and human brains that continues to generate neurons throughout adulthood, therefore an attack on this region could adversely affect memory creation.
Neural stem cells within the hippocampus experienced the most significant alterations due to Treg depletion. These cells are crucial as they possess the capability to differentiate into various specialized brain cell types. However, lacking Tregs significantly hindered their ability to transition into other cell types. Their activity diminished or completely ceased, and they began to undergo cell death.
The absence of Tregs appeared to leave an enduring “scar” in the hippocampus, resulting in a lasting functional impairment.
in short-term memory development, the investigators stated. Treg-deficient subjects encountered difficulties with short-term memory that lingered even months after their Tregs were returned to standard levels.
But how precisely do Tregs manage to regulate other cells?
In a concluding series of experiments, the investigators discovered that in the brains of healthy rodents, Tregs maintain control over inflammation-promoting immune cells by competing for a common resource — a growth factor named IL-2. Once Tregs were eliminated, other immune cells could absorb this cellular energy, proliferate rapidly, and generate inflammatory proteins.
A path to comprehend and address neurodegenerative disorders
Inflammation has long been associated with various neurodegenerative disorders, so the subsequent inquiry, according to Mathis, is: Do Tregs in human brains contribute to mitigating the inflammation that propels these degenerative developments?
Mathis’ team is presently investigating this precise question utilizing a mouse model of Alzheimer’s disease. Concurrently, they are collaborating with colleagues in the neuropathology and neurosurgery divisions at Massachusetts General Hospital to explore this phenomenon in human brains affected by Alzheimer’s.
In recent times, Treg-based treatments have sparked enthusiasm regarding the potential of employing these cells in an organ-specific or tissue-targeted manner to treat immune-mediated disorders. These initiatives encompass lab-modified Tregs (CAR-Tregs and T-cell receptor Tregs) as well as the formulation of therapeutic molecules that could precisely adjust Treg functionality in a targeted and localized manner.
“Gaining a clear understanding of how Tregs execute their protective roles may someday assist us in developing therapies that enhance their activity to modulate a broad spectrum of disease mechanisms,” Mathis remarked.
Other contributors included Elisa Cintado, Alec J. Walker, Teshika Jayewickreme, Felipe A. Pinho-Ribeiro, Quentin Richardson, Ruaidhrí Jackson, Isaac M. Chiu, Christophe Benoist, Beth Stevens, and José Luís Trejo.
The investigation outlined in this article received backing from the JPB Foundation, the Spanish Ministry of Science and Innovation, the National Institutes of Health, the NIH Director’s New Innovator Award, and the Crohn’s & Colitis Foundation. Additional assistance was rendered through HHMI, the Cure Alzheimer’s Fund, and via a predoctoral fellowship from the Spanish Ministerio de Economia y Competitividad (Ministry of Economy and Competitiveness).