“`html
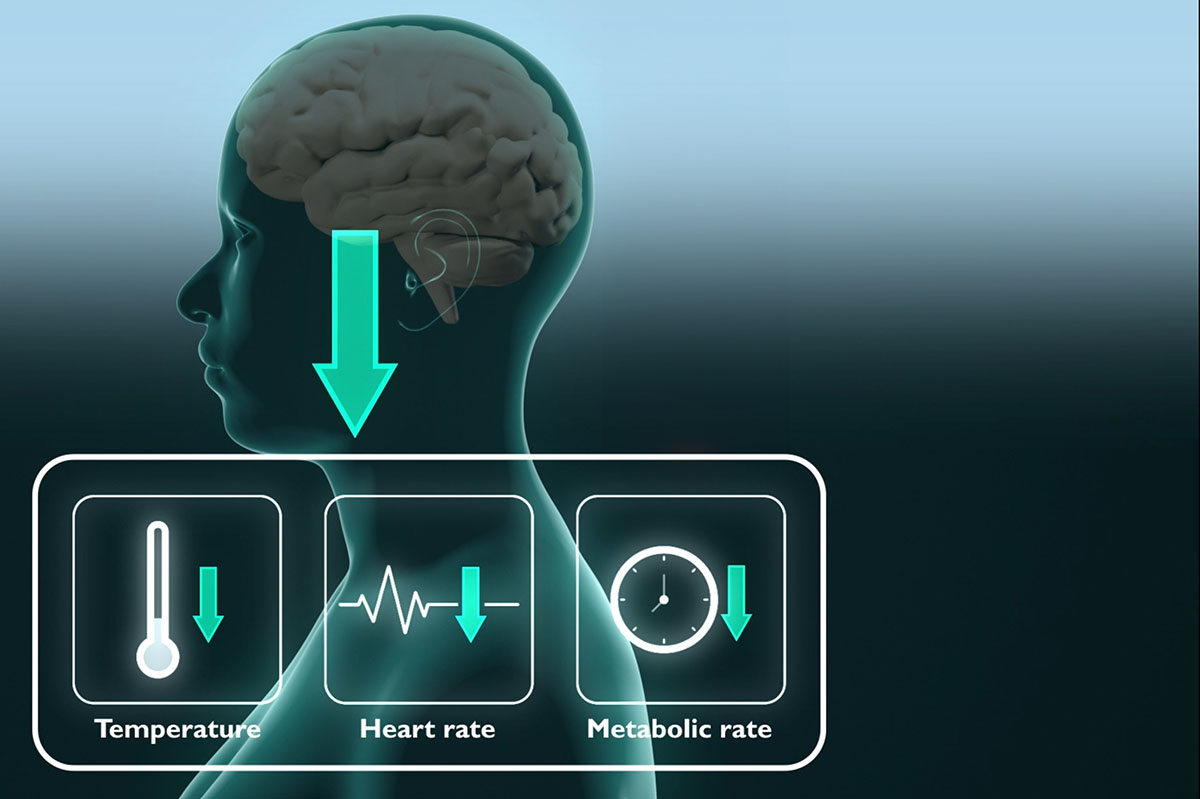
Nature frequently serves as the supreme example for scientific exploration. For almost a century, researchers have been striving to replicate the capabilities of certain mammals and birds to endure harsh environmental challenges for short or prolonged durations by entering a state of torpor, during which their body temperature and metabolic rates decrease, enabling energy and heat conservation.
Drawing inspiration from the natural world, Hong Chen, a professor of biomedical engineering at the McKelvey School of Engineering and neurosurgery at WashU Medicine, along with a multidisciplinary team, successfully induced a reversible torpor-like condition in mice and rats by employing focused ultrasound to activate the hypothalamus preoptic area within the brain, which plays a crucial role in regulating body temperature and metabolism.
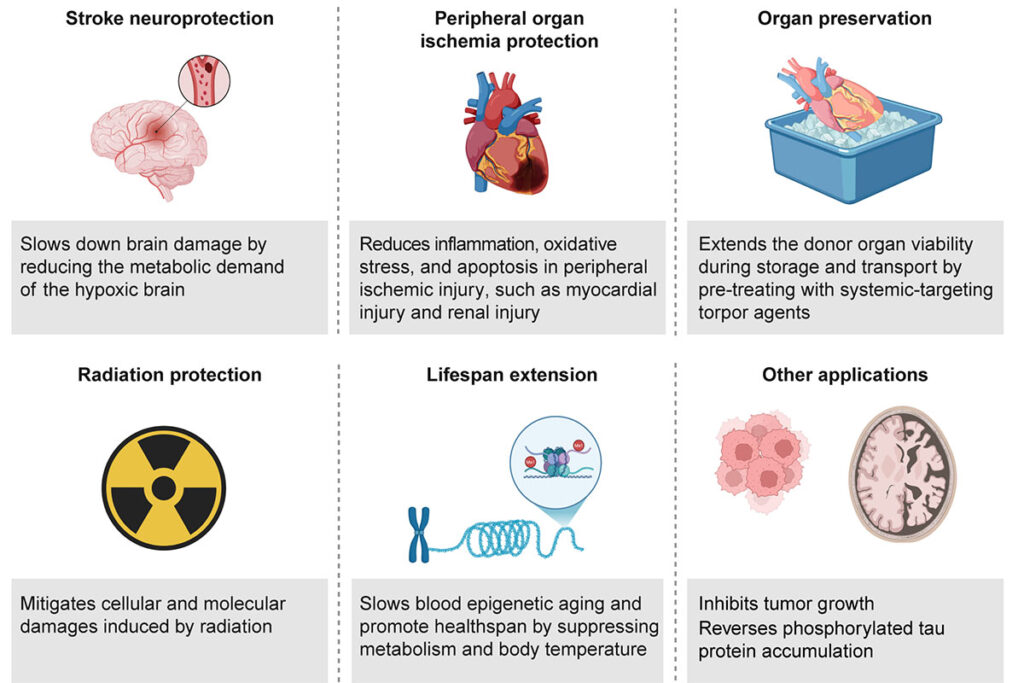
The research team is now focused on converting induced, or synthetic, torpor into potential applications for humans, such as during instances of diminished blood flow to tissues or organs, preserving organs for transplant or shielding against radiation exposure during space travel.
Traditional medical approaches aim to augment energy availability, such as restoring cerebral blood flow post-stroke. Conversely, synthetic torpor aims to lessen energy consumption.
“The ability of synthetic torpor to manage overall metabolic activity holds the potential to revolutionize medicine by providing innovative approaches for medical treatments,” Chen stated in a Perspectives article released in Nature Metabolism on July 31.
Synthetic torpor has demonstrated success in preclinical models using specific medications and targeted neural circuit interventions, yet challenges remain in adapting these techniques for human application. Earlier human trials involving hydrogen sulfide were abruptly halted due to safety issues.
“Our hurdles include addressing metabolic differences between animals and humans, selecting the appropriate medication dosage, and devising methods to maintain a reversible torpor-like state,” remarked Wenbo Wu, a doctoral student in biomedical engineering in Chen’s lab and primary author of the Perspectives document, a partnership between Chen’s group and Genshiro Sunagawa from the RIKEN Center for Biosystems Dynamics Research in Japan. “Collaboration among researchers, medical professionals, and ethicists will be essential for developing secure, effective, and scalable solutions for synthetic torpor to materialize as a viable medical intervention.”
Chen’s team concentrated on the neural circuit with their induced torpor method in mice. The team featured Yaoheng (Mack) Yang, who was a postdoctoral research associate in her lab at Washington University in St. Louis and is now an assistant professor of biomedical engineering at the University of Southern California.
They created a portable ultrasound transducer to activate neurons in the hypothalamus preoptic area. Upon stimulation, the mice exhibited a decrease in body temperature of roughly 3 degrees Celsius for about an hour. Furthermore, the mice’s metabolism shifted from utilizing both carbohydrates and fat for energy to relying solely on fat, a distinctive aspect of torpor, while their heart rates dropped by approximately 47%, all at room temperature.
“Ultrasound stands out as the sole noninvasive energy mechanism capable of safely penetrating the skull and accurately targeting deep-seated brain structures,” Chen remarked.
Chen and her colleagues suggest that synthetic torpor presents a promising therapeutic avenue with additional potential, including the inhibition of tumor proliferation and the possible creation of new treatments for tau protein-related diseases, like Alzheimer’s disease. Nevertheless, much remains to be understood regarding how brain areas, peripheral organs, and cellular pathways collaborate in metabolic suppression and awakening. Researchers should also investigate long-term risks and prospective side effects while advocating for further preclinical investigations and technological advancements that will enable a dual strategy, incorporating modulation of neural circuits linked to hypometabolism and influencing peripheral metabolic mechanisms through systemic approaches, such as pharmacological interventions or peripheral neuromodulation.
“Synthetic torpor is no longer merely a theoretical notion — it has emerged as a field with the capability to redefine medicine,” Chen highlighted. “Integrating fundamental neuroscience, bioengineering, and translational medicine will be crucial to surmounting present obstacles and advancing synthetic torpor toward practical applications. This field could evolve from a scientific curiosity into a human reality through interdisciplinary collaboration.”
Wu W, Sunagawa GA, Chen H. Synthetic torpor: Advancing metabolic regulation for medical innovations. Nature Metabolism, July 31, 2025. DOI: 10.1038/s42255-025-01345-3
This research was supported by the National Institutes of Health (DP1DK143574 R01NS12846) and JSPS Grant-in-Aid for Scientific Research (A) 24H00604), as well as JSPS Grant-in-Aid for Transformative Research Areas (A) 23H04941.
Initially published on the McKelvey Engineering website
The article Synthetic torpor has potential to redefine medicine first appeared on The Source.
“`