“`html
When cells are robust, we don’t anticipate them to abruptly transition between cell types. A skin cell on your hand won’t spontaneously transform into a brain cell, and vice versa. This is due to epigenetic memory, which facilitates the regulation of various genes to “lock in” throughout a cell’s existence. A breakdown of this memory can result in ailments, such as cancer.
Historically, researchers have believed that epigenetic memory toggles genes either “on” or “off” — either entirely activated or completely suppressed, akin to a steadfast Lite-Brite pattern. However, MIT engineers have discovered that the reality is much more nuanced.
In a fresh investigation published today in Cell Genomics, the team reveals that a cell’s memory is determined not by binary switching but via a more gradual, dimmer-like dial of gene expression.
The investigators conducted experiments where they adjusted the expression of a single gene at varying levels across different cells. While standard belief would suggest that the gene should ultimately toggle on or off, the researchers observed that the gene’s initial expression endured: Cells with gene expression positioned on a spectrum between on and off maintained this intermediate state.
The findings imply that epigenetic memory — the mechanism through which cells retain gene expression and “recall” their identity — is not binary, but rather analog, facilitating a range of gene expression and corresponding cell identities.
“Our discovery opens the door for the possibility that cells commit to their final identity by securing genes at specific levels of gene expression rather than merely toggling them on and off,” states study author Domitilla Del Vecchio, professor of mechanical and biological engineering at MIT. “The implication is that there may be numerous more cell types in our bodies than we currently recognize, which could have vital roles and potentially underpin healthy or diseased conditions.”
The study’s lead authors from MIT are Sebastian Palacios and Simone Bruno, along with additional co-authors.
Beyond binary
Every cell carries the same genome, which can be viewed as the essential component for life. As a cell develops, it differentiates into one type or another through the expression of its genomic genes. Some genes are activated, while others are suppressed. The combination directs a cell towards one identity as opposed to another.
A mechanism of DNA methylation, where specific molecules attach to the genes’ DNA, aids in securing their expression in place. DNA methylation helps a cell “remember” its distinctive pattern of gene expression, ultimately determining the cell’s identity.
Del Vecchio’s team at MIT combines mathematics and genetic engineering to comprehend cellular molecular processes and to modify cells with new capabilities. In previous research, her group was investigating DNA methylation and methods to secure the expression of specific genes in ovarian cells.
“The conventional understanding held that DNA methylation played a role in securing genes in either an on or off state,” Del Vecchio remarks. “We believed this to be the established dogma. However, we began observing results that contradicted that notion.”
While many of the cells in their experiment displayed an all-or-nothing expression of genes, a noteworthy number of cells seemed to freeze genes in an intermediate state — neither completely on nor off.
“We found that there was a spectrum of cells expressing any level between on and off,” Palacios remarks. “And we wondered, how is this feasible?”
Shades of blue
In their latest study, the team aimed to determine whether the intermediate gene expression they observed was an anomaly or a more established characteristic of cells that has gone unnoticed until now.
“It could be that scientists overlooked cells that don’t exhibit a clear commitment, assuming this was merely a transient state,” Del Vecchio states. “But in reality, these intermediate cell types may be permanent states that could have significant roles.”
To validate their hypothesis, the researchers conducted experiments with hamster ovarian cells — a cell line frequently utilized in laboratory settings. In each cell, an engineered gene was initially calibrated to a different expression level. The gene was fully activated in some cells, entirely inactivated in others, and adjusted somewhere in between for the remaining cells.
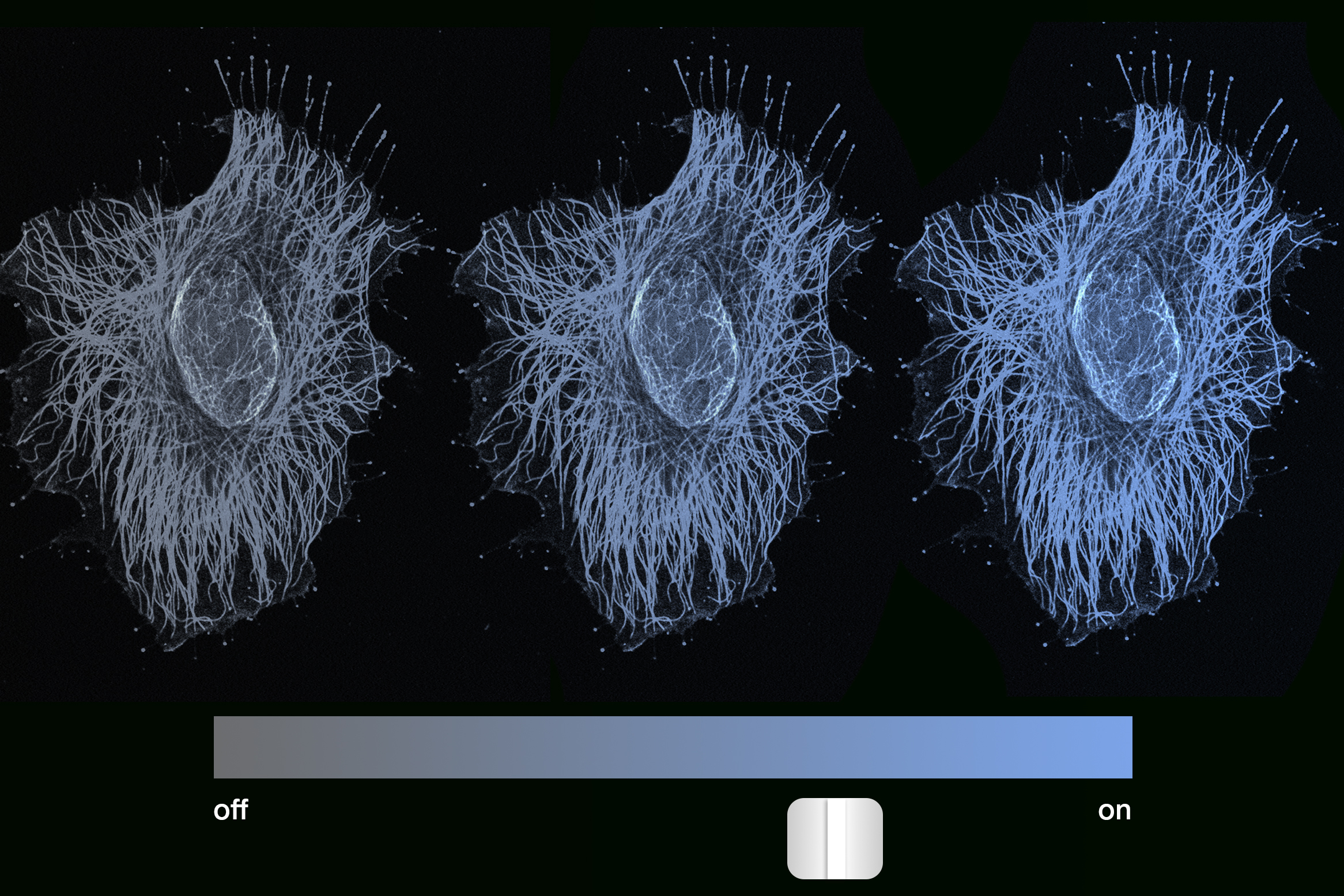
The team coupled the engineered gene with a fluorescent marker that illuminates with brightness proportional to the gene’s expression level. The researchers briefly introduced an enzyme that triggers the gene’s DNA methylation, a natural gene-locking process. They subsequently observed the cells over five months to see if the modification would secure the genes at their intermediate expression levels, or if the genes would trend towards fully activated or inactivated states before locking in.
“Our fluorescent marker is blue, and we observe cells glowing across the entire spectrum, from very bright blue, to progressively dimmer, to no blue at all,” Del Vecchio comments. “Every intensity level is maintained over time, which indicates that gene expression is graded, or analog, and not binary. We were extremely surprised, as we anticipated that after such an extensive period, the gene would shift to be either fully on or off, but it did not.”
The discoveries pave new paths in engineering more intricate artificial tissues and organs by adjusting the expression of specific genes in a cell’s genome, similar to a dial on a radio, rather than a switch. The outcomes also complicate the understanding of how a cell’s epigenetic memory functions to define its identity. It opens up potential treatments for cellular modifications, like those found in therapy-resistant tumors, in a more precise manner.
“Del Vecchio and colleagues have elegantly demonstrated how analog memory arises through chemical changes to the DNA itself,” states Michael Elowitz, professor of biology and biological engineering at the California Institute of Technology, who was not involved in this study. “Consequently, we can now envision repurposing this natural analog memory mechanism, evolved through nature, in the field of synthetic biology, where it could aid in programming permanent and precise multicellular behaviors.”
“One of the factors that enables complexity in humans is epigenetic memory,” Palacios remarks. “And we discover that it is not as we previously thought. For me, that’s truly astonishing. I believe we’re going to discover that this analog memory is relevant for numerous different processes across biology.”
This research received partial support from the National Science Foundation, MODULUS, and a Vannevar Bush Faculty Fellowship through the U.S. Office of Naval Research.
“`