“`html
Rotavirus leads to severe dehydrating diarrhea in infants and toddlers, causing over 128,500 fatalities annually worldwide, even with extensive immunization initiatives. Though rotavirus is more common in underdeveloped nations, a decrease in vaccination rates in the United States has led to a rise in cases in recent times.
Recent findings from Washington University School of Medicine in St. Louis have pinpointed a crucial mechanism that allows rotavirus to invade cells. The investigators discovered that interrupting this process in tissue cultures and in mice halted infection. This revelation paves the way for new therapeutic strategies to combat rotavirus and other pathogens utilizing similar infection tactics.
The findings were published in PNAS.
“Rotavirus takes the lives of infants and children, young individuals who never had a chance,” remarked Siyuan Ding, an associate professor of molecular microbiology at WashU Medicine. “This is why we aim to create effective therapies, despite already having available vaccines. Not every child gets vaccinated, and this virus is exceptionally contagious. Once a child contracts the virus, there’s currently no cure; we can only alleviate the symptoms.”
Enzyme as an entry key
To propose a potential treatment, Ding and his team concentrated on aspects of the body’s cells that could be utilized to defend against viral infection. This approach, according to Ding, may be less likely to induce drug resistance compared to targeting the virus directly and has the potential to address multiple afflictions as it is founded on common infection pathways rather than disease-specific characteristics.
When a rotavirus particle penetrates the cell’s outer membrane, it doesn’t instantly become free to infect the cell. Instead, the virus is contained within a minuscule cell compartment known as an endosome.
The researchers discovered an enzyme within cells, termed fatty acid 2-hydroxylase (FA2H), that is vital for allowing rotavirus to escape endosomes and fully infect cells. By employing advanced gene-editing methods, they excised the FA2H gene from human cells and observed that viruses remained ensnared in endosomes and could not replicate efficiently. In essence, disabling FA2H obstructed infection from the outset.
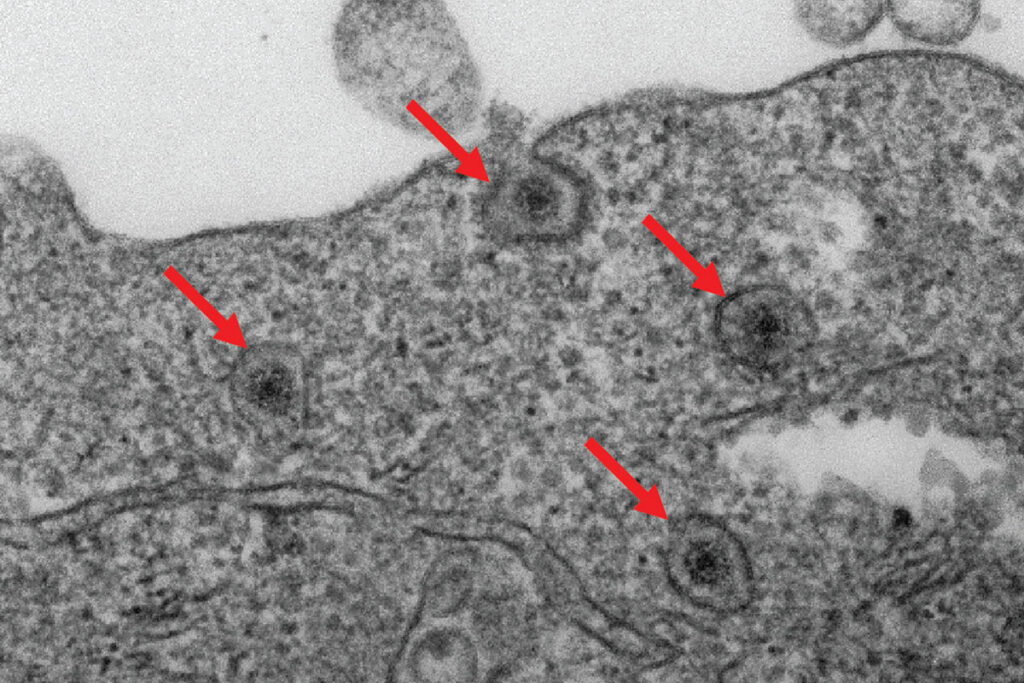
To validate these findings in animal models, the scientists engineered genetically modified mice lacking the FA2H enzyme in the cells lining the small intestine. These mice exhibited significantly fewer symptoms when infected with rotavirus in contrast to normal mice, underscoring the significance of FA2H in viral infections.
Unlike vaccines that generally prompt the body to generate antibodies that prevent pathogens from infiltrating cells initially, disabling FA2H intervenes in the natural trajectory of infection to establish a supplementary line of host-centric cellular defense against rotavirus and related infections.
“Viruses depend on their hosts, so we are thwarting infection by inhibiting their use of the host’s cellular mechanisms,” Ding stated. “We had limited understanding of how this enzyme, FA2H, functioned until this research, but we are now observing that the same mechanism assists other pathogens, like the Junín virus and Shiga toxin, hinting at a shared ‘entry code’ employed by various disease-causing agents.”
With Ding and his collaborators having recognized this pathway as a broadly exploitable entry mechanism, they can commence trials of medications that mimic the effect of FA2H gene alteration.
Li E, Zang R, Kawagishi T, Zhang W, Iyer K, Hou G, Zeng Q, Meganck RM, Ross SR, Wang X, Su X, Ding S. Fatty acid 2-hydroxylase facilitates rotavirus uncoating and endosomal escape. PNAS. September 3, 2025. DOI: https://www.pnas.org/doi/10.1073/pnas.2511911122
This research is backed by NIH R01 AI150796 and R01 AI159290. The material is solely the accountability of the authors and does not necessarily reflect the official views of the NIH.
About Washington University School of Medicine
WashU Medicine is a global leader in academic medicine, covering biomedical research, patient care, and educational initiatives with over 3,000 faculty members. Its National Institutes of Health (NIH) research funding portfolio ranks second largest among U.S. medical schools and has increased by 83% since 2016. Together with institutional investments, WashU Medicine allocates well over $1 billion annually towards basic and clinical research innovation and training. Its faculty practice consistently ranks in the top five nationally, with over 2,000 physician faculty operating at 130 locations. WashU Medicine clinicians solely staff Barnes-Jewish and St. Louis Children’s hospitals — the academic hospitals of BJC and Siteman Cancer Center, a collaboration between BJC HealthCare and WashU Medicine, and the only National Cancer Institute-designated comprehensive cancer center in Missouri. Physicians at WashU Medicine also provide care at BJC’s community hospitals in the region. With a remarkable history in MD/PhD training, WashU Medicine recently allocated $100 million for scholarships and curriculum enhancement for its medical students and boasts outstanding training programs across all medical specialties, as well as physical therapy, occupational therapy, and audiology and communication sciences.
Originally published on the WashU Medicine website
The post Researchers find key to stopping deadly infection appeared first on The Source.
“`