Researchers at the McGovern Institute for Brain Research at MIT and the Broad Institute of MIT and Harvard have redesigned a compact RNA-guided enzyme they discovered in bacteria into an effective, programmable editor of human DNA.
The protein they developed, known as NovaIscB, can be tailored to make precise modifications to the genetic code, regulate the activity of specific genes, or perform other editing functions. Due to its small size, which facilitates delivery to cells, the creators of NovaIscB assert it is a promising candidate for developing gene therapies aimed at treating or preventing diseases.
The research was spearheaded by Feng Zhang, the James and Patricia Poitras Professor of Neuroscience at MIT, who is also an investigator at the McGovern Institute and the Howard Hughes Medical Institute, and a core member of the Broad Institute. Zhang and his team shared their open-access findings this month in the journal Nature Biotechnology.
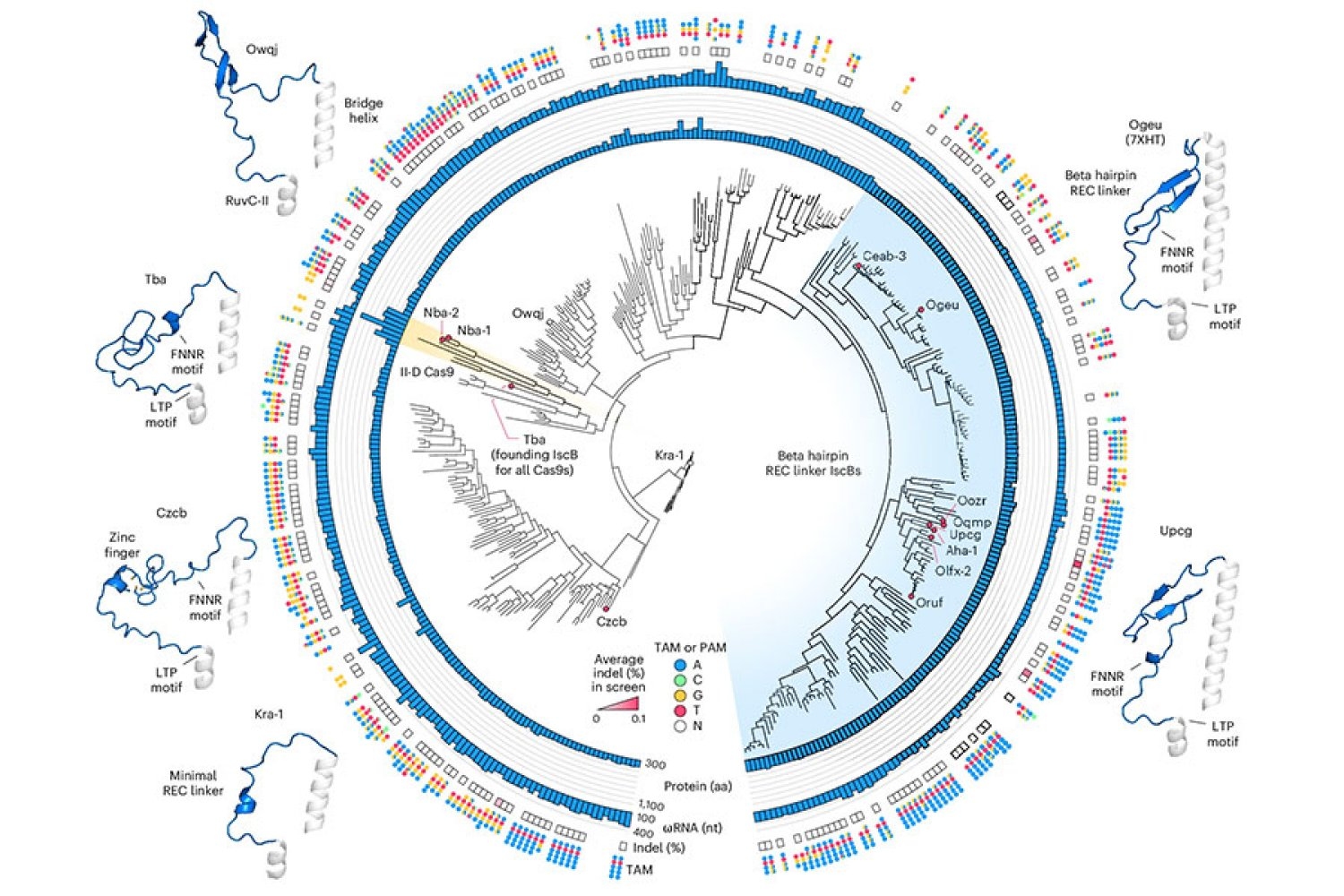
NovaIscB is derived from a bacterial DNA-cleaving enzyme that belongs to a class of proteins known as IscBs, which Zhang’s laboratory identified in 2021. IscBs represent a type of OMEGA system, the evolutionary predecessors to Cas9, which is part of the bacterial CRISPR system that Zhang and others have transformed into powerful genome-editing tools. Similar to Cas9, IscB enzymes cleave DNA at locations specified by an RNA guide. By reprogramming that guide, scientists can redirect the enzymes to target sequences of their choice.
The team was attracted to IscBs not only because they share critical characteristics with CRISPR’s DNA-cleaving Cas9 but also because they are one-third its size. This compactness would be beneficial for potential gene therapies: smaller tools are easier to introduce into cells, and a diminutive enzyme would offer researchers greater flexibility to experiment, potentially adding new functionalities without creating tools that are too large for clinical application.
From their initial investigations of IscBs, researchers in Zhang’s lab recognized that some members of the family could cut DNA targets in human cells. However, none of the bacterial proteins showed sufficient efficacy for therapeutic use: the team needed to modify an IscB to ensure it could effectively edit targets in human cells without disturbing the rest of the genome.
To initiate that engineering process, Soumya Kannan, a graduate student in Zhang’s lab who is now a junior fellow at the Harvard Society of Fellows, and postdoc Shiyou Zhu first searched for an IscB that would serve as a solid starting point. They experimented with approximately 400 different IscB enzymes found in bacteria. Ten demonstrated the ability to edit DNA in human cells.
Even the most effective of those would still need enhancement to become a valuable genome-editing tool. The challenge would be increasing the enzyme’s activity, but solely at the sequences designated by its RNA guide. If the enzyme became more active indiscriminately, it could cleave DNA in unintended areas. “The critical aspect is to balance the enhancement of both activity and specificity simultaneously,” explains Zhu.
Zhu adds that bacterial IscBs are directed to their target sequences by relatively short RNA guides, which makes it challenging to confine the enzyme’s activity to a specific section of the genome. If an IscB could be engineered to accommodate a longer guide, it would be less prone to acting on sequences beyond its intended target.
To enhance IscB for human genome editing, the team utilized insights that graduate student Han Altae-Tran, who is now a postdoc at the University of Washington, had gathered about the variation among bacterial IscBs and their evolutionary progress. For instance, the researchers noted that IscBs which functioned in human cells included a segment they termed REC, which was missing in other IscBs. They hypothesized that the enzyme might require that segment to interact effectively with DNA in human cells. Upon closer examination of the region, structural models indicated that by slightly expanding a portion of the protein, REC could also allow IscBs to recognize longer RNA guides.
Based on these observations, the team tested swapping in portions of REC domains from various IscBs and Cas9s, assessing how each modification affected the protein’s functionality. Guided by their comprehension of how IscBs and Cas9s interact with both DNA and their RNA guides, the researchers made further changes, intending to optimize both efficacy and specificity.
Ultimately, they produced a protein they named NovaIscB, which exhibited over 100 times more activity in human cells than the IscB they initially began with, while also demonstrating excellent specificity for its targets.
Kannan and Zhu constructed and screened hundreds of new IscBs before settling on NovaIscB — each alteration they made to the original protein was intentional. Their efforts were informed by their team’s understanding of the natural evolution of IscBs, as well as forecasts of how each modification would impact the protein’s structure, informed by an artificial intelligence tool called AlphaFold2. In contrast to traditional methods of making random alterations to a protein and screening their effects, this strategic engineering approach significantly expedited the team’s ability to identify a protein with the desired characteristics.
The team illustrated that NovaIscB serves as an effective scaffold for a variety of genome editing tools. “It functions biochemically very similarly to Cas9, which simplifies the integration of tools that were already refined with the Cas9 scaffold,” Kannan states. With various modifications, the researchers utilized NovaIscB to change specific letters of the DNA code in human cells and to alter the activity of targeted genes.
Significantly, NovaIscB-based tools are compact enough to be easily encapsulated within a single adeno-associated virus (AAV) — the vector most frequently used to safely deliver gene therapy to patients. Due to their larger size, tools developed using Cas9 can necessitate a more complex delivery strategy.
Demonstrating NovaIscB’s therapeutic potential, Zhang’s team created a tool called OMEGAoff that adds chemical tags to DNA to reduce the activity of specific genes. They programmed OMEGAoff to suppress a gene involved in cholesterol regulation, then employed AAV to transport the system to the livers of mice, resulting in sustained reductions in cholesterol levels in the blood of those animals.
The team anticipates that NovaIscB can be applied to target genome editing tools to most human genes and looks forward to observing how other laboratories implement this new technology. They also hope others will adopt their evolution-guided framework for rational protein engineering. “Nature possesses such diversity, and its systems have different strengths and weaknesses,” Zhu remarks. “By understanding that natural diversity, we can enhance the systems we are striving to engineer.”
This research was supported, in part, by the K. Lisa Yang and Hock E. Tan Center for Molecular Therapeutics at MIT, Broad Institute Programmable Therapeutics Gift Donors, Pershing Square Foundation, William Ackman, Neri Oxman, the Phillips family, and J. and P. Poitras.