“`html
Globally, 20 percent of youngsters are not fully vaccinated, resulting in 1.5 million fatalities among children each year due to diseases that vaccinations can prevent. Approximately half of these underimmunized children received at least one vaccine dose but failed to finish the complete vaccination schedule, while the remainder received no vaccinations whatsoever.
To simplify the process for children to obtain all their vaccines, MIT researchers are endeavoring to create microparticles capable of releasing their contents weeks or months after administration. This innovation could facilitate vaccines that require only a single injection, with multiple doses dispersed at various intervals.
In a study published today in the journal Advanced Materials, the researchers demonstrated that they could utilize these particles to administer two doses of the diphtheria vaccine — one released immediately and the other two weeks later. Mice that received this vaccine produced as many antibodies as those that were given two separate doses spaced two weeks apart.
The researchers now aspire to prolong these intervals, which could render the particles beneficial for administering childhood vaccines that consist of multiple doses over several months, like the polio vaccine.
“The overarching aim of this work is to create vaccines that enhance accessibility to immunization — particularly for children residing in regions where health care facilities are hard to reach. This includes rural areas of the United States as well as certain parts of the developing world where medical infrastructure is limited,” states Ana Jaklenec, a principal investigator at MIT’s Koch Institute for Integrative Cancer Research.
Jaklenec and Robert Langer, the David H. Koch Institute Professor at MIT, serve as the senior authors of the study. Linzixuan (Rhoda) Zhang, an MIT graduate student who recently earned her PhD in chemical engineering, is the lead author of the paper.
Self-boosting vaccines
In recent years, Jaklenec, Langer, and their associates have been focusing on vaccine delivery particles made from a polymer known as PLGA. In 2018, they revealed their capability to use these types of particles to release two doses of the polio vaccine approximately 25 days apart.
One limitation of PLGA is that as the particles gradually degrade within the body, the immediate environment may turn acidic, potentially harming the vaccine enclosed within the particles.
The MIT team is currently seeking methods to resolve this issue in PLGA particles and is also investigating alternative materials that would create a less acidic environment. In the current study, led by Zhang, the researchers opted to concentrate on another type of polymer, referred to as polyanhydride.
“The objective of this study was to advance the field by exploring innovative strategies to tackle significant challenges, particularly those linked to pH sensitivity and antigen degradation,” says Jaklenec.
Polyanhydrides, biodegradable polymers that Langer designed for drug delivery over 40 years ago, are highly hydrophobic. This characteristic means that as the polymers slowly dissolve within the body, the breakdown products barely dissolve in water and produce a much less acidic environment.
Polyanhydrides typically consist of chains formed from two different monomers that can be assembled in a vast array of potential combinations. For this study, the researchers crafted a library of 23 polymers, which varied from one another based on the chemical structures of the monomer building blocks and the ratio of the two monomers incorporated into the final product.
The researchers assessed these polymers based on their resistance to temperatures of at least 104 degrees Fahrenheit (40 degrees Celsius, or slightly above body temperature) and their stability throughout the microparticle formation process.
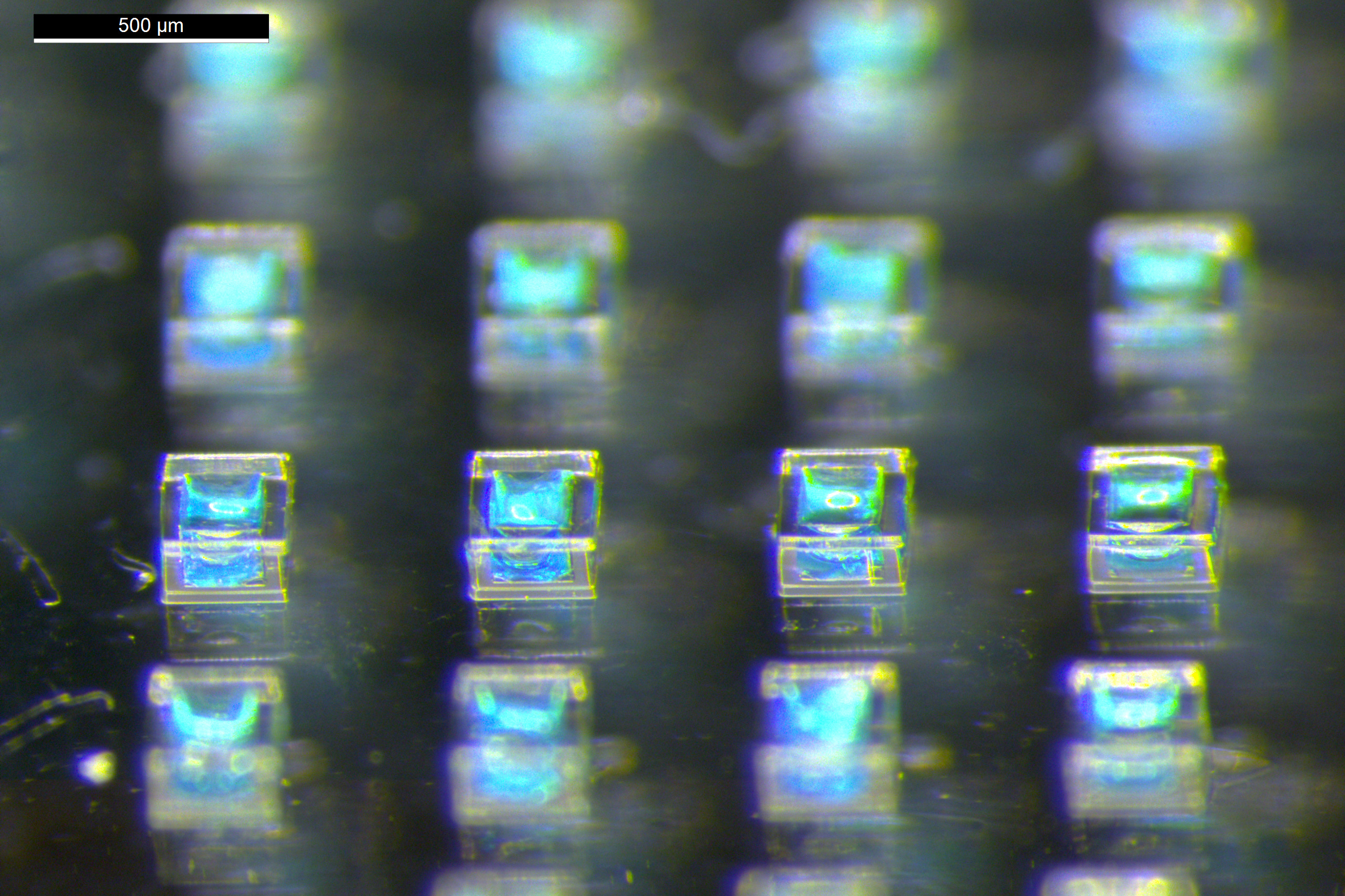
To create the particles, the researchers devised a technique known as stamped assembly of polymer layers, or SEAL. Initially, they utilize silicon molds to shape cup-like particles that can be filled with the vaccine antigen. Then, a top made from the same polymer is applied and sealed via heat. Polymers that were too fragile or did not seal adequately were filtered out, resulting in six leading candidates.
The researchers then utilized those polymers to engineer particles that would release diphtheria vaccine two weeks after injection, administering these to mice alongside a vaccine that was released immediately. Four weeks following the initial injection, these mice exhibited antibody levels comparable to those of mice that received two doses two weeks apart.
Extended release
As part of their research, the researchers also developed a machine-learning model to investigate the elements that influence the duration it takes for the particles to degrade once inside the body. These elements encompass the type of monomers involved in the material, the monomer ratio, the molecular weight of the polymer, and the loading capacity, or how much vaccine can be contained within the particle.
Employing this model, the researchers efficiently evaluated nearly 500 potential particles and forecasted their release time. They experimented with several of these particles in controlled buffers and demonstrated that the model’s predictions were valid.
In forthcoming research, this model could additionally assist scientists in formulating materials that would release their contents over extended intervals — spanning months or even years. This approach could be advantageous for administering various childhood vaccines, which necessitate multiple doses over several years.
“If we aim to extend this to longer durations, say over a month or even more, we certainly have strategies to achieve this, such as increasing the molecular weight or the hydrophobicity of the polymer. We might also consider some cross-linking. These modifications would alter the polymer’s chemistry to decelerate the release kinetics or enhance the particle’s retention time,” Zhang remarks.
The researchers are now eager to investigate the use of these delivery particles for additional types of vaccines. The particles could also be of significant value for administering other forms of medications that are sensitive to acidity and require multiple doses, they indicate.
“This technology holds extensive promise for single-injection vaccines but could also be adjusted to deliver small molecules or other biologics needing durability or multiple administrations. Furthermore, it can accommodate drugs with pH sensitivities,” Jaklenec concludes.
The research was partially funded by the Koch Institute Support (core) Grant from the National Cancer Institute.
“`