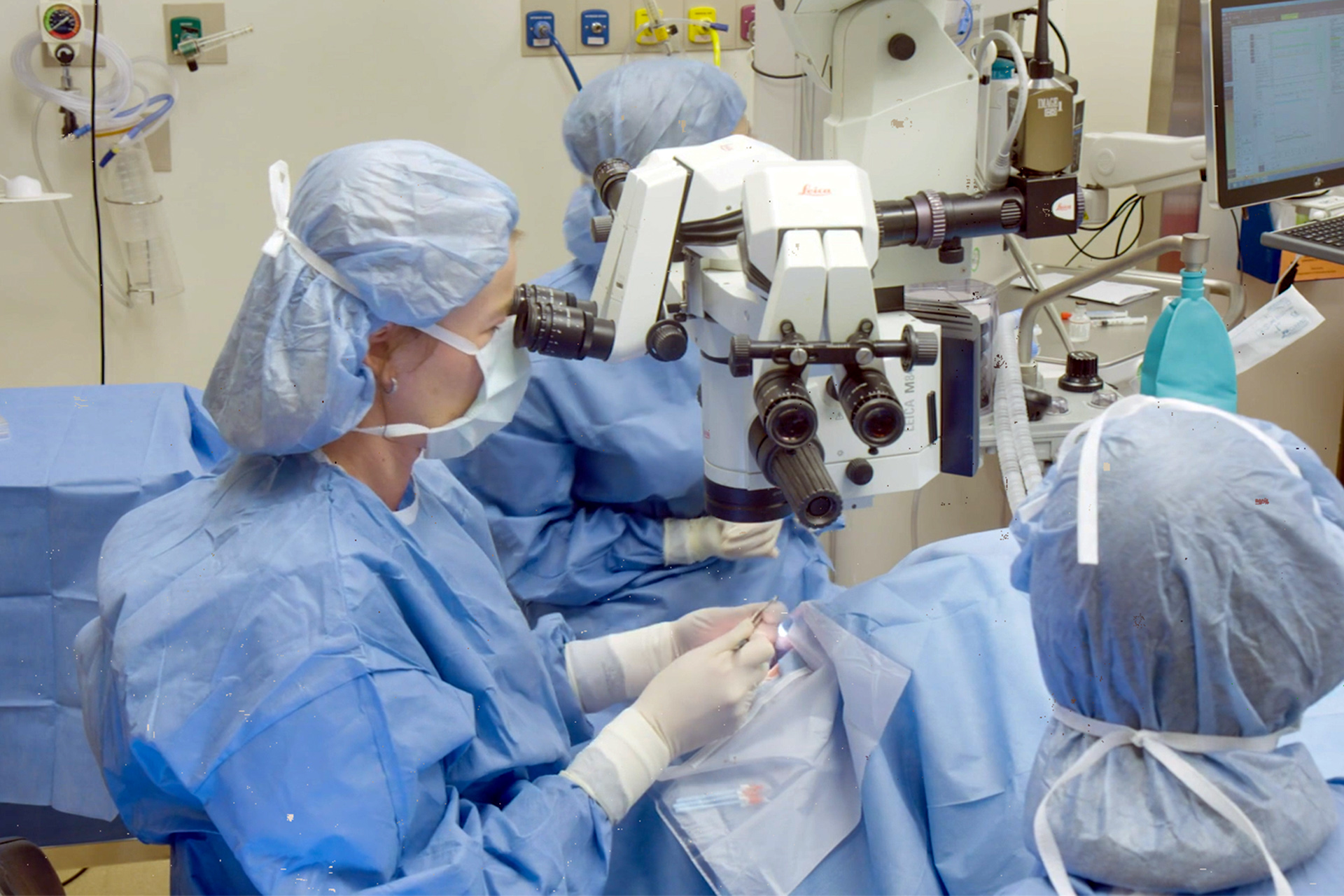
Ula Jurkunas conducts the first CALEC surgery at Mass Eye and Ear.
Image courtesy of MGH
Health
New possibilities for repairing eye damage once deemed untreatable
Stem cell therapy effectively restores cornea’s surface during clinical trials
A clinical trial spearheaded by Mass Eye and Ear involving a technique that used stem cells from a healthy eye to implant them into a damaged eye successfully restored corneal surfaces in 14 patients tracked over 18 months.
The stem cell therapy targeting severe corneal injuries — termed cultivated autologous limbal epithelial cells, or CALEC — was innovated at Mass Eye and Ear. The method involves harvesting stem cells from a healthy eye via biopsy, cultivating them into a cellular tissue graft through a groundbreaking production process lasting two to three weeks, and then surgically implanting the graft into the eye suffering from corneal damage.
“Our initial trial indicated that CALEC was safe and the treatment was feasible,” stated principal investigator Ula Jurkunas, associate director of the Cornea Service at Mass Eye and Ear and professor of ophthalmology at Harvard Medical School. “Currently, we possess this newfound evidence affirming that CALEC achieves over 90 percent effectiveness in restoring the cornea’s surface, which significantly benefits individuals with corneal damage previously thought to be untreatable.”
The cornea is the transparent, outermost layer of the eye. Its peripheral region, the limbus, contains a substantial quantity of healthy stem cells known as limbal epithelial cells, which preserve the eye’s smooth surface.
When an individual sustains a corneal injury, such as a chemical burn, infection, or other trauma, it can deplete the limbal epithelial cells, which cannot regenerate. The resulting limbal stem cell deficiency leaves the eye with an irreparably damaged surface, preventing it from undergoing a corneal transplant, which is the current standard of care for vision recovery. Those with these injuries frequently endure persistent pain and visual challenges.
National Eye Institute
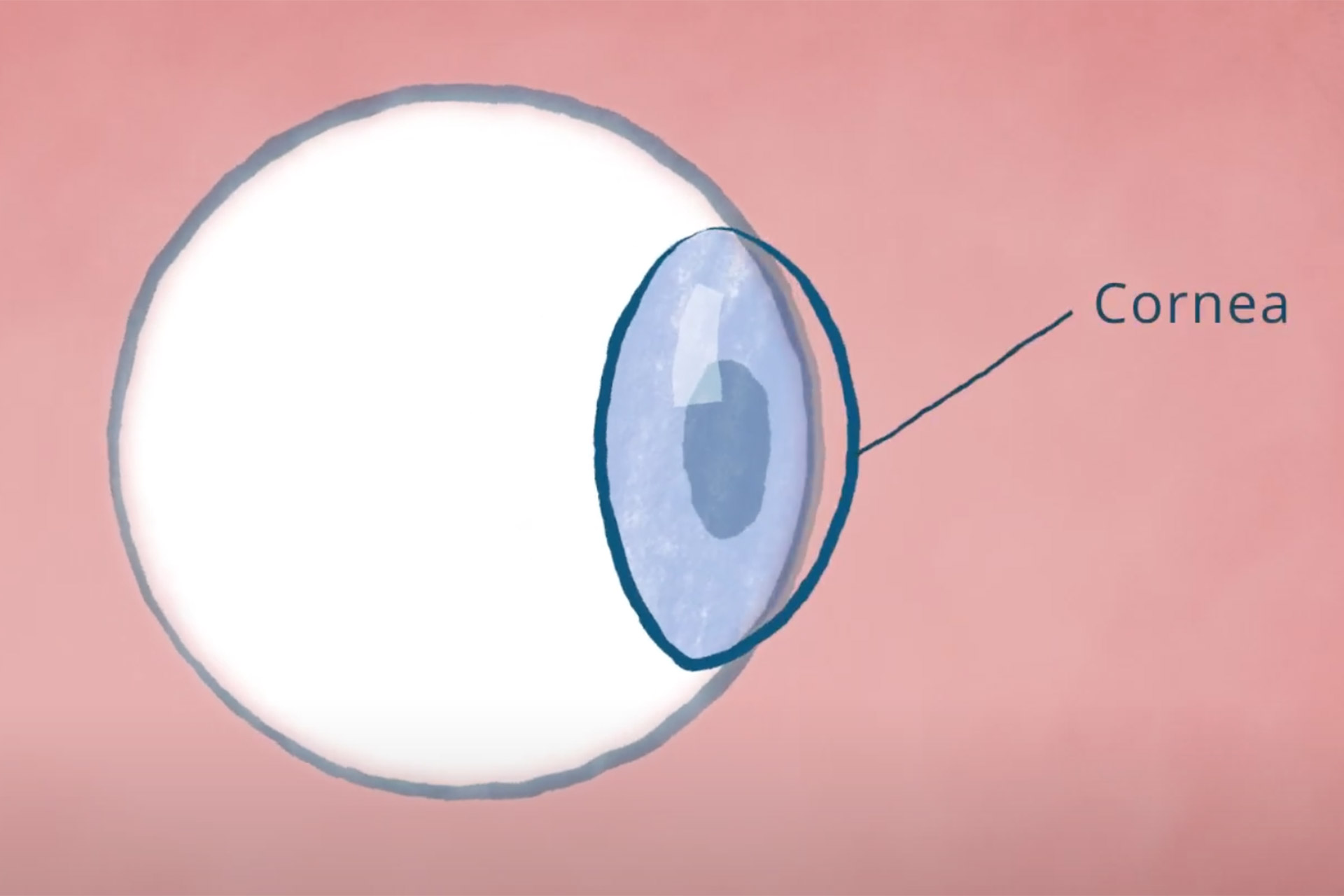
How the cornea gets destroyed beyond repair
This necessity prompted Jurkunas and Reza Dana, director of the Cornea Service at Mass Eye and Ear, to investigate a novel methodology for regenerating limbal epithelial cells. Almost twenty years later, after preliminary studies and partnerships with researchers at Dana-Farber and Boston Children’s, it became achievable to consistently produce CALEC grafts that satisfied the rigorous quality criteria required for human transplantation. The clinical trial received approval from the U.S. Food and Drug Administration and Mass General Brigham Institutional Review Board, with the first patient treated in 2018 at Mass Eye and Ear. The successful execution of the trial was accomplished through close collaboration between Jurkunas’ surgical team and the cell manufacturing facility at Dana-Farber.
A constraint of this technique is that it is essential for the patient to possess only one affected eye, allowing a biopsy to be done to procure initial material from the unaffected normal eye.
“Our future aspiration is to establish an allogeneic manufacturing process using limbal stem cells sourced from a normal cadaveric donor eye,” remarked Jerome Ritz of Dana-Farber Cancer Institute’s Connell and O’Reilly Families Cell Manipulation Core Facility, where the stem cell grafts are produced. “This will ideally broaden the application of this method and enable treatment for patients with damage to both eyes.”
Researchers demonstrated that the procedure completely restored the cornea in 50 percent of participants during their three-month evaluation, and the complete success rate rose to 79 percent and 77 percent during their 12- and 18-month assessments, respectively. With two participants achieving partial success at 12 and 18 months, the overall success rates were 93 percent and 92 percent at 12 and 18 months. Three participants underwent a second transplant, with one achieving complete success by the conclusion of the follow-up visit. A further evaluation of the procedure’s effect on vision revealed varying degrees of enhancement in visual acuity among all 14 patients.
CALEC displayed
A robust safety profile was observed, with no significant incidents reported in either the donor or recipient eyes. An isolated adverse event, specifically a bacterial infection, was noted in a participant eight months post-transplant, attributed to prolonged contact lens wear. Other adverse incidents were minor and swiftly resolved after the procedures were conducted.
The procedure is still in the experimental phase and is not currently provided at Mass Eye and Ear or any hospitals in the United States, and further research will be necessary before the treatment can be submitted for federal endorsement.
This trial marks the initial human investigation of a stem cell therapy backed by the National Eye Institute, part of the National Institutes of Health, and was the pioneering stem cell therapy for ocular applications within the U.S. Additional research partners include Jia Yin at Mass Eye and Ear; Myriam Armant of Boston Children’s Hospital; and the JAEB Center for Health Research.
Looking ahead, subsequent CALEC studies should encompass a greater patient population across multiple sites, with extended follow-up periods and a randomized control framework.
“We believe this research justifies more trials that can pave the way for FDA approval,” expressed Jurkunas. “While we take pride in advancing a new treatment from laboratory to clinical trials, our primary goal has always been to ensure that patients nationwide can access this effective therapy.”
This study is supported by the National Eye Institute of the National Institutes of Health.
Disclosures: CALEC is currently patent-pending. Jurkunas and Dana hold financial stakes in OcuCell, a company focused on developing live cell-based therapies for eye disorders. Armant is part of the scientific advisory board for OcuCell. Ritz receives research funding from Kite/Gilead, Novartis, and Oncternal, and participates on Scientific Advisory Boards for Astraveus, Garuda Therapeutics, Smart Immune, Tolerance Bio, and TriArm Therapeutics. The other authors declare no conflicts of interest.