The realm of tissue engineering seeks to mimic the architecture and functionality of actual biological tissues. This manufactured tissue holds significant promise for applications in disease simulation, pharmaceutical exploration, and implantable grafts.
3D bioprinting, which employs living cells, biocompatible substances, and growth factors to construct three-dimensional tissue and organ frameworks, has surfaced as a pivotal instrument in this domain. Up to now, one of the most prevalent methods for bioprinting utilizes additive manufacturing techniques and digital models, layering 2D strands of bio-inks, comprised of cells in a soft gel, into a supportive bath, layer-by-layer, to create a 3D form. While these methods facilitate the creation of intricate architectures with features that are challenging to fabricate by hand, existing strategies exhibit constraints.
“A significant limitation of current 3D bioprinting methods is their failure to incorporate process control strategies that minimize flaws in printed tissues. Integrating process control could enhance tissue inter-reproducibility and optimize resource efficiency, such as minimizing material wastage,” explains Ritu Raman, the Eugene Bell Career Development Chair of Tissue Engineering and an assistant professor of mechanical engineering.
She adds, “Considering the diverse range of available 3D bioprinting tools, there exists a considerable necessity to create process optimization strategies that are modular, efficient, and user-friendly.”
This necessity prompted Raman to collaborate with Professor Bianca Colosimo from the Polytechnic University of Milan, widely recognized as Polimi. Colosimo recently finished a sabbatical at MIT, hosted by John Hart, Class of 1922 Professor, co-director of MIT’s Initiative for New Manufacturing, director of the Center for Advanced Production Technologies, and head of the Department of Mechanical Engineering.
“Artificial Intelligence and data mining are already transforming our everyday lives, and their influence will be even more significant in the developing field of 3D bioprinting, and in manufacturing overall,” states Colosimo. During her MIT sabbatical, she worked with Raman and her team to jointly develop a solution that marks a preliminary step toward smart bioprinting.
“This solution is currently accessible in both our laboratories at Polimi and MIT, functioning as a twin platform to share data and findings across various settings and laying the groundwork for numerous cooperative projects in the future,” Colosimo remarks.
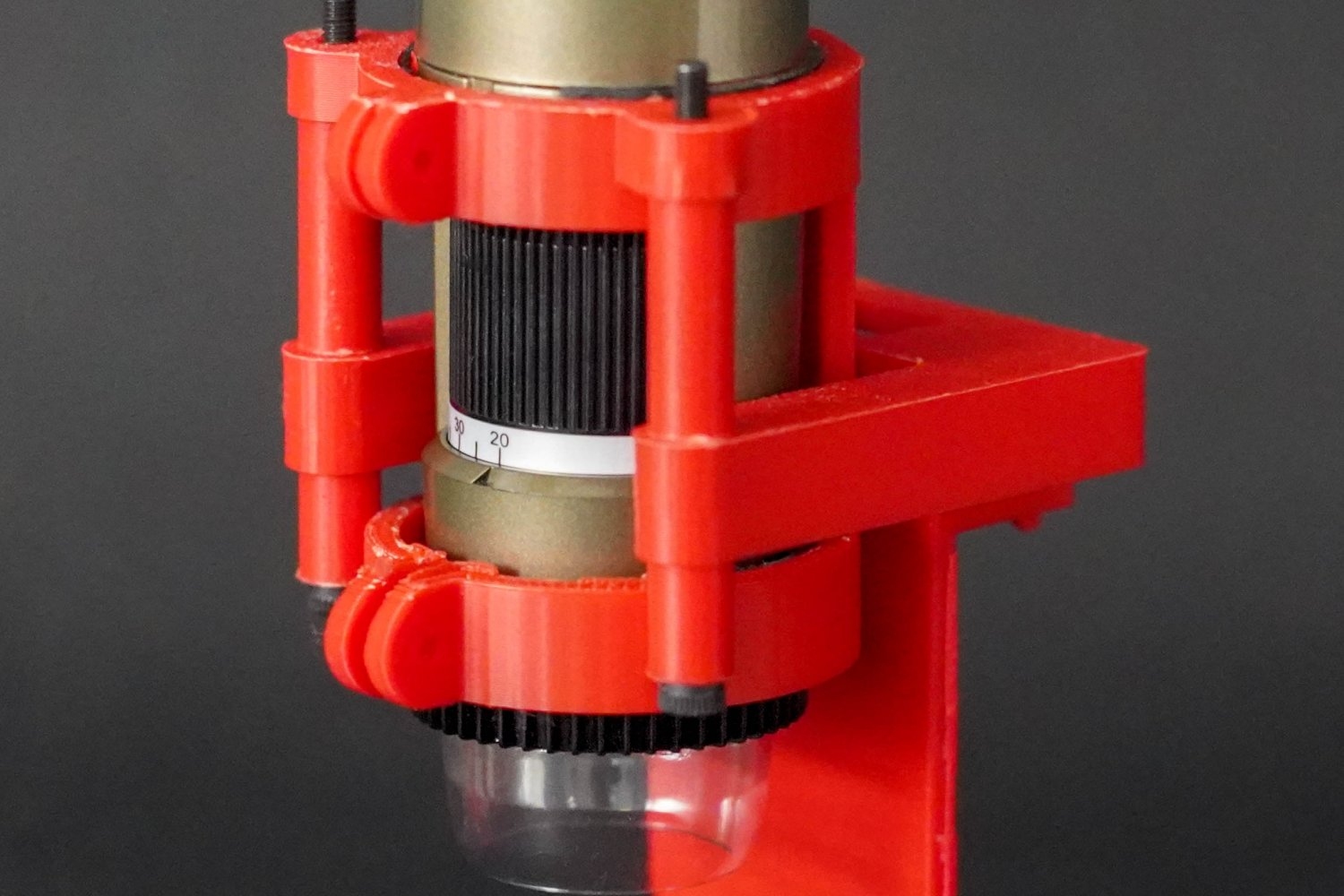
A recent publication by Raman, Colosimo, and lead authors Giovanni Zanderigo, a Rocca Fellow at Polimi, and Ferdows Afghah of MIT released this week in the journal Device introduces an innovative technique that tackles this issue. The team constructed and validated a modular, cost-effective, and printer-agnostic monitoring method that includes a compact tool for imaging layer-by-layer. In their approach, a digital microscope records high-resolution images of tissues during printing and swiftly compares them to the intended design utilizing an AI-based image analysis pipeline.
“This technique allowed us to rapidly detect print imperfections, such as applying excessive or inadequate bio-ink, thereby assisting us in determining optimal print parameters for a variety of different materials,” states Raman. “The method is a low-cost — less than $500 — scalable, and versatile solution that can be easily employed on any standard 3D bioprinter. Here at MIT, the monitoring platform has already been integrated into the 3D bioprinting facilities in The SHED. Beyond MIT, our research provides a concrete pathway toward improved reproducibility, enhanced sustainability, and automation in the field of tissue engineering. This research could positively influence human health by elevating the quality of the tissues we manufacture to investigate and treat debilitating injuries and diseases.”
The authors note that the new technique is more than just a monitoring apparatus. It also serves as a groundwork for intelligent process regulation in embedded bioprinting. By enabling real-time evaluation, adaptive correction, and automated parameter adjustments, the researchers foresee that the approach can elevate reproducibility, minimize material waste, and expedite process optimization for practical applications in tissue engineering.