Transforming one kind of cell into another — for instance, changing a skin cell into a neuron — can be achieved through a procedure that necessitates inducing the skin cell into a “pluripotent” stem cell, followed by differentiating it into a neuron. Researchers at MIT have now designed a more straightforward approach that circumvents the stem cell phase, allowing for the direct transformation of a skin cell into a neuron.
Utilizing mouse cells, the researchers formulated a conversion technique that is remarkably efficient, capable of generating over 10 neurons from a single skin cell. If applied to human cells, this method could facilitate the production of substantial quantities of motor neurons, which might be harnessed to assist patients suffering from spinal cord injuries or conditions that hinder mobility.
“We successfully achieved yields sufficient to explore whether these cells could serve as viable candidates for cell replacement therapies, which we anticipate they might be. That’s the potential of these types of reprogramming technologies,” states Katie Galloway, the W. M. Keck Career Development Professor in Biomedical Engineering and Chemical Engineering.
As an initial step towards utilizing these cells as a treatment, the researchers demonstrated their capability to generate motor neurons and transplant them into the brains of mice, where they assimilated with the surrounding tissue.
Galloway is the principal author of two publications detailing the novel method, which are published today in Cell Systems. MIT graduate student Nathan Wang is the primary author of both studies.
From skin to neurons
Around 20 years prior, scientists in Japan discovered that by administering four transcription factors to skin cells, they could encourage them to become induced pluripotent stem cells (iPSCs). Comparable to embryonic stem cells, iPSCs have the ability to be differentiated into various other cell types. While this technique is effective, it requires several weeks, and a significant portion of the cells fail to completely transition into mature cell types.
“Frequently, one of the obstacles in reprogramming is that cells can become stalled in intermediate phases,” Galloway explains. “Hence, we are employing direct conversion, which allows us to move directly from a somatic cell to a motor neuron without passing through an iPSC intermediary.”
Galloway’s research team and others have previously exhibited this kind of direct conversion, yet with very low yields — less than 1 percent. In her earlier research, Galloway applied a mixture of six transcription factors along with two additional proteins that promote cell proliferation. Each of those eight genes was introduced using a distinct viral vector, complicating the assurance that each was expressed at the appropriate level in each cell.
In the first of the new Cell Systems publications, Galloway and her students reported a method to optimize the process so that skin cells can be transformed into motor neurons using merely three transcription factors, in conjunction with the two genes that propel cells into a highly proliferative state.
Using mouse cells, the researchers began with the original six transcription factors and experimented by sequentially omitting them, until they identified a combination of three — NGN2, ISL1, and LHX3 — capable of successfully completing the conversion to neurons.
After reducing the gene count to three, the researchers could utilize a single modified virus to convey all three, enabling them to confirm that each cell expresses each gene at the appropriate levels.
Moreover, using a different virus, the researchers also introduced genes coding for p53DD and a mutated version of HRAS. These genes prompt the skin cells to multiply extensively before starting their conversion to neurons, resulting in a significantly higher yield of neurons, approximately 1,100 percent.
“If transcription factors are expressed at extremely high levels in nonproliferative cells, the reprogramming rates would be quite low, but hyperproliferative cells are more receptive. It’s as if they’ve been primed for conversion, making them much more receptive to the levels of transcription factors,” Galloway remarks.
The researchers also established a slightly different combination of transcription factors that enabled them to conduct the same direct conversion using human cells, although with a lower efficiency rate — estimated between 10 and 30 percent. This procedure requires roughly five weeks, which is somewhat quicker than first converting the cells to iPSCs and then differentiating them into neurons.
Implanting cells
Once the researchers pinpointed the optimal gene combination to deliver, they began exploring the most effective delivery methods, which was the emphasis of the second Cell Systems publication.
They tested three different delivery viruses and discovered that a retrovirus achieved the highest efficiency rate of conversion. Additionally, decreasing the cell density in culture improved the overall yield of motor neurons. This optimized procedure, which takes about two weeks in mouse cells, resulted in a yield exceeding 1,000 percent.
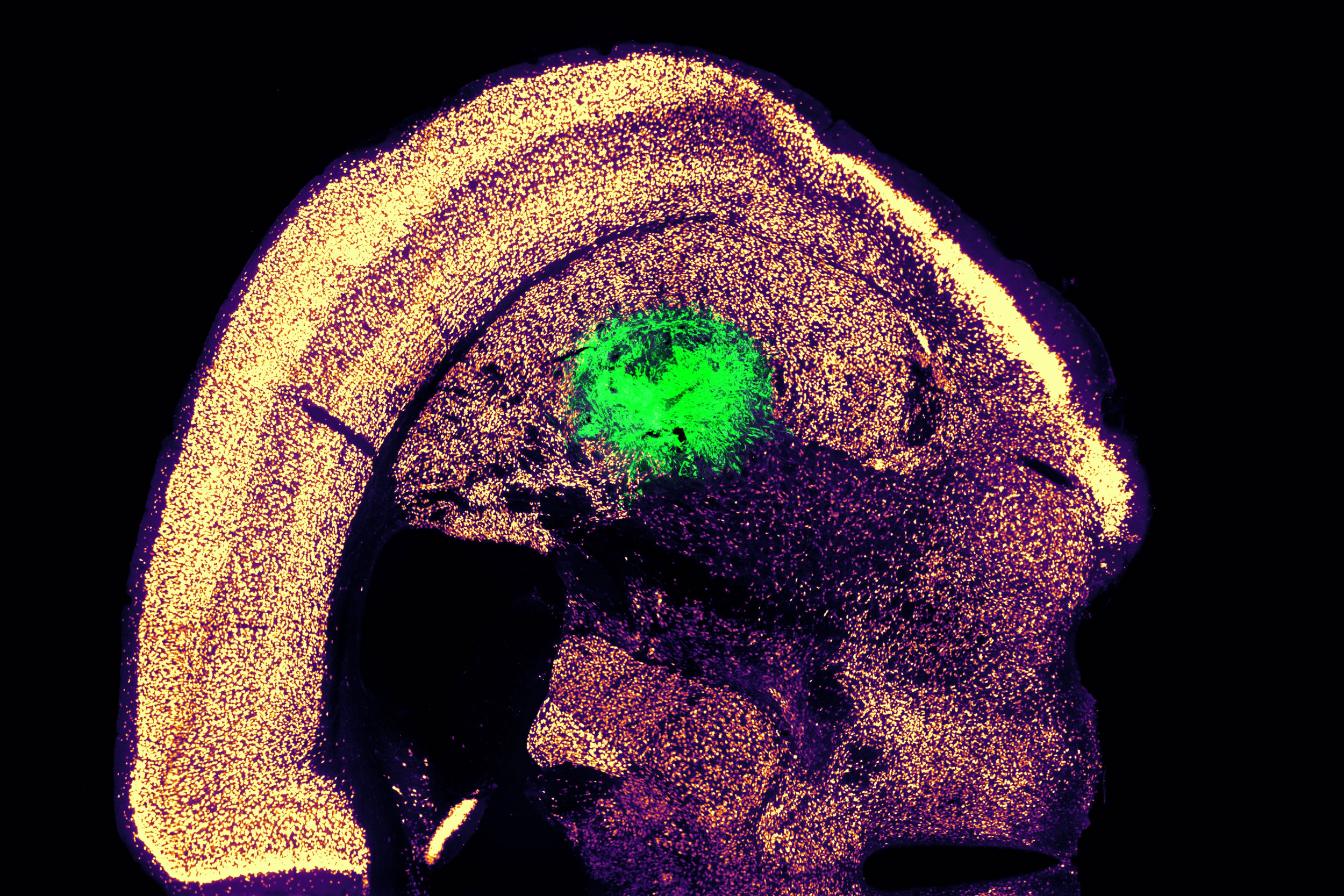
Collaborating with colleagues at Boston University, the researchers then examined whether these motor neurons could be effectively integrated into mice. They delivered the cells to a brain region known as the striatum, which is essential for motor control and various other functions.
After two weeks, the researchers found that many of the neurons had survived and appeared to be establishing connections with other brain cells. When maintained in a dish, these cells exhibited measurable electrical activity and calcium signaling, indicating their ability to communicate with other neurons. The researchers are now keen to investigate the potential of transplanting these neurons into the spinal cord.
The MIT team also aims to enhance the efficiency of this conversion process for human cells, which could facilitate the production of large quantities of neurons usable for addressing spinal cord injuries or conditions that compromise motor control, such as ALS. Clinical trials using neurons derived from iPSCs for ALS treatment are currently underway, but increasing the availability of cells for such therapies could simplify their testing and development for broader applications in humans, Galloway notes.
The study received funding from the National Institute of General Medical Sciences and the National Science Foundation Graduate Research Fellowship Program.