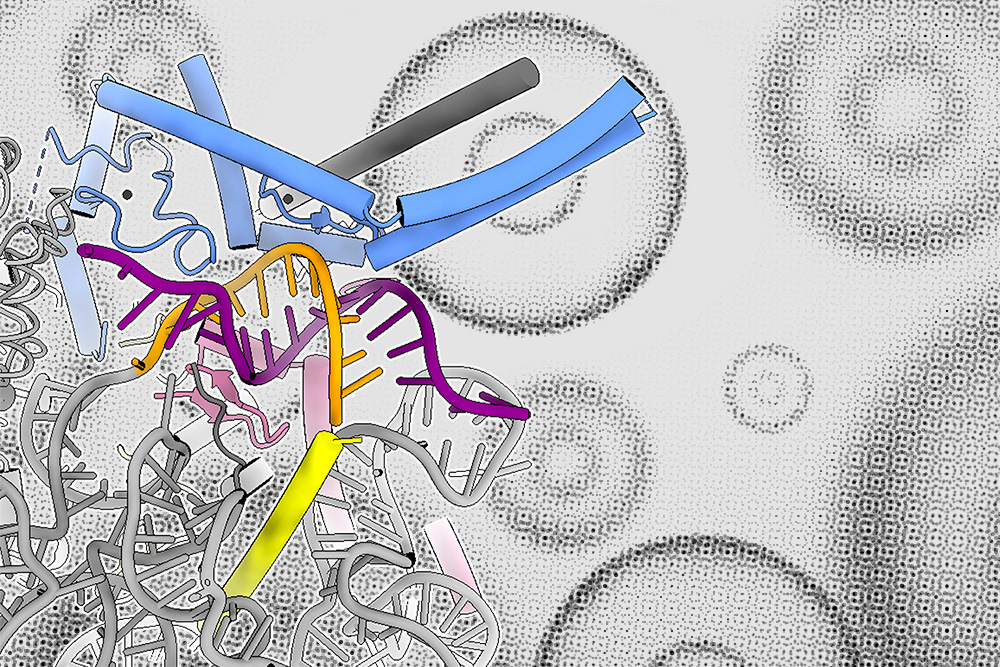
RNA splicing is a vital cellular mechanism essential for gene expression. Once genes are transcribed from DNA into messenger RNA, segments of the RNA that do not code for proteins, referred to as introns, are removed, and the coding segments are reconnected.
This procedure is regulated by a substantial protein-RNA assembly known as the spliceosome. Researchers at MIT have recently uncovered an additional layer of regulation that aids in identifying which locations on the messenger RNA molecule the spliceosome will focus on.
The research team revealed that this form of regulation, which seems to affect the expression of nearly half of all human genes, is present throughout the animal kingdom, as well as in plant life. The results indicate that the regulation of RNA splicing, a process fundamental to gene expression, is more intricate than previously understood.
“Splicing in more advanced organisms, such as humans, is more intricate than it is in simpler model organisms like yeast, even though it remains a highly conserved molecular process. There are nuances in the human spliceosome that enhance its ability to process particular introns more effectively. One potential benefit of such a system is that it may facilitate more sophisticated types of gene regulation,” states Connor Kenny, a graduate student at MIT and the primary author of the research.
Christopher Burge, the Uncas and Helen Whitaker Professor of Biology at MIT, is the principal author of the paper, which is published today in Nature Communications.
Constructing proteins
RNA splicing, identified in the late 1970s, permits cells to meticulously regulate the composition of the mRNA transcripts that relay the instructions for synthesizing proteins.
Each mRNA transcript comprises coding regions, called exons, and noncoding regions, named introns. They also incorporate sites that function as signals for splicing locations, enabling the cell to compile the proper sequence for a specific protein. This mechanism allows a single gene to generate multiple proteins; over evolutionary timeframes, splicing can also alter the dimensions and content of genes and proteins as various exons are added or excluded.
The spliceosome, which assembles on introns, consists of proteins and noncoding RNAs known as small nuclear RNAs (snRNAs). The initial phase of spliceosome formation involves an snRNA molecule dubbed U1 snRNA attaching to the 5’ splice site located at the intron’s start. Until now, it was believed that the binding strength between the 5’ splice site and the U1 snRNA was the primary factor determining whether an intron would be excised from the mRNA transcript.
In the current study, the MIT team uncovered that a protein family called LUC7 also contributes to establishing whether splicing will take place, but solely for a subset of introns — in human cells, as much as 50 percent.
Prior to this investigation, it was recognized that LUC7 proteins associate with U1 snRNA, but their exact role was unclear. There are three distinct LUC7 proteins present in human cells, and Kenny’s experiments demonstrated that two of these proteins specifically interact with one type of 5’ splice site, which the researchers designated as “right-handed.” A third human LUC7 protein engages with a different type, referred to as “left-handed.”
The researchers established that approximately half of human introns possess either a right- or left-handed site, while the remaining half seemingly do not interact with LUC7 proteins for control. This regulatory mechanism appears to introduce an additional layer of regulation that enhances the efficiency of specific intron removal, according to the researchers.
“The study reveals the existence of these two distinct subclasses of 5’ splice sites that can be regulated separately,” says Kenny. “Some core splicing mechanisms are indeed more intricate than we previously recognized, indicating that further scrutiny is warranted regarding our assumptions about these highly conserved molecular processes.”
“Intricate splicing machinery”
Previous research has indicated that mutations or deletions of one of the LUC7 proteins interacting with right-handed splice sites are associated with blood malignancies, including around 10 percent of acute myeloid leukemias (AMLs). In this analysis, the researchers found that AML cases that lost a copy of the LUC7L2 gene exhibit ineffective splicing of right-handed splice sites. These cancers also displayed a similar metabolic alteration seen in earlier studies.
“Comprehending how the absence of this LUC7 protein in certain AMLs alters splicing could assist in designing treatments that leverage these splicing variations to combat AML,” notes Burge. “Additionally, there are small molecule medications for other ailments, such as spinal muscular atrophy, that stabilize the interaction between U1 snRNA and specific 5’ splice sites. Thus, the insight that particular LUC7 proteins affect these interactions at specific splice sites may enhance the precision of this class of small molecules.”
Collaborating with a laboratory led by Sascha Laubinger, a professor at Martin Luther University Halle-Wittenberg, the researchers discovered that introns in plants also possess right- and left-handed 5’ splice sites regulated by Luc7 proteins.
The team’s analysis implies that this type of splicing evolved in a common ancestor of plants, animals, and fungi, but was lost in fungi shortly after they diverged from plants and animals.
The researchers now intend to conduct a more detailed analysis of the structures formed through the interactions of Luc7 proteins with mRNA and the remainder of the spliceosome, which may assist them in understanding more intricately how various forms of Luc7 attach to different 5’ splice sites.
This study received funding from the U.S. National Institutes of Health as well as the German Research Foundation.