Over 30 million individuals in the United States depend on implanted medical devices such as prosthetic limbs, pacemakers, and others to enhance their living standards. However, inserting any external object into the body also involves the risk of introducing lethal fungal infections.
Recent findings from scientists at the University of Georgia reveal how a transcription regulatory protein linked to the fungal pathogen Candida albicans orchestrates a variety of genes to infect medical devices within the human body.
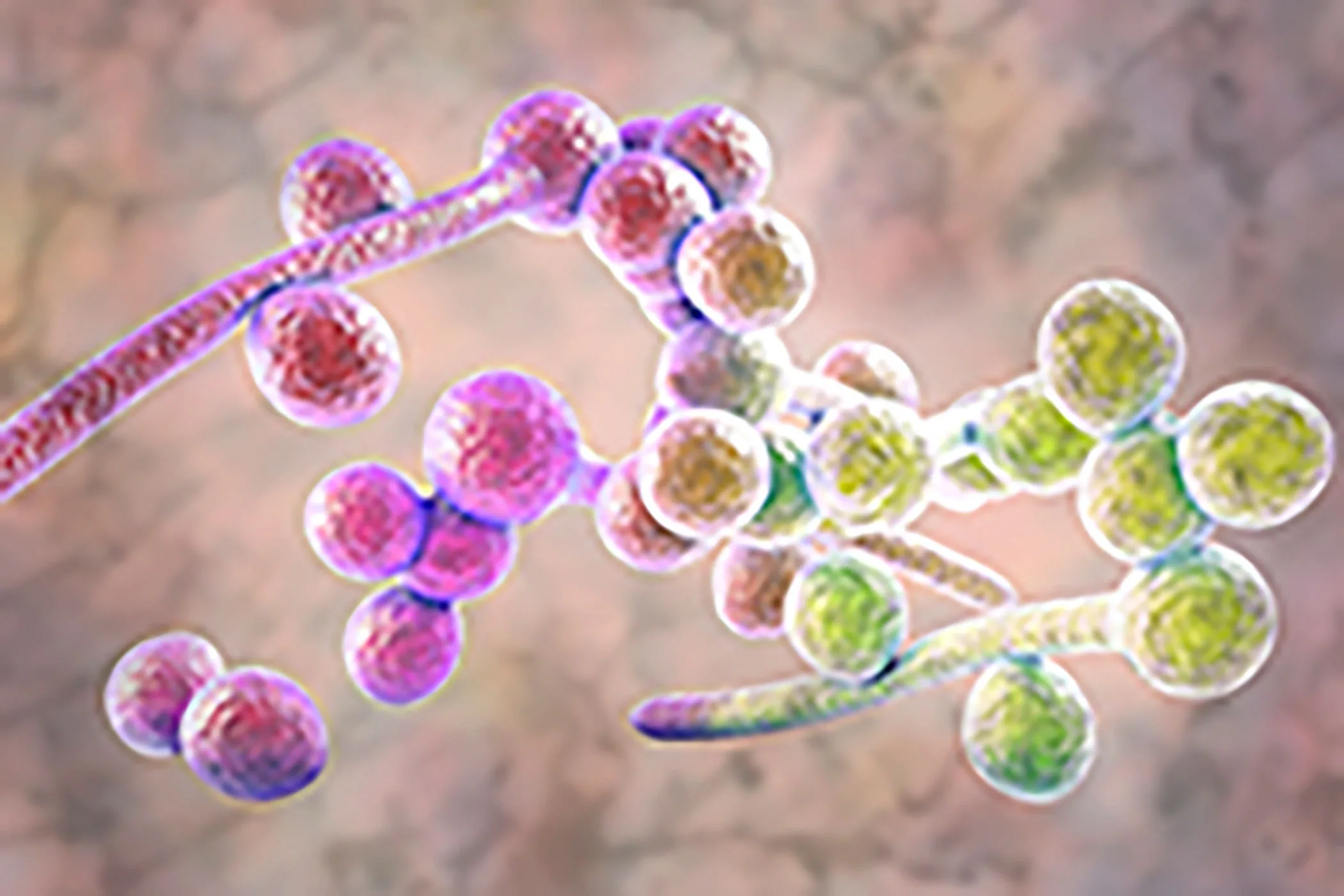
Candida albicans is generally a benign yeast commonly present in humans. Yet, an excessive growth of this fungus can result in yeast infections, thrush, or even potentially fatal invasive candidiasis, which may lead to organ failure.
Medical devices that could potentially harbor infections include catheters, pacemakers, artificial heart valves, and prosthetic joints.
“Overall infection rates differ … but typically range from 500,000 to 1 million annually in the U.S.,” stated Aaron Mitchell, lead author of the study and Distinguished Research Professor in UGA’s Franklin College of Arts and Sciences microbiology department.
Biofilms on medical devices present infection hazards
Medical devices offer an attractive surface for microorganisms to attach, and once these biofilms develop, they are difficult to penetrate.
Biofilms emit cells that infect deeper tissues and often show little response to antifungal treatments.
In the context of Candida albicans, biofilm formation necessitates the coordination of gene sets responsible for adherence, cell development and maturation, and growth in low-oxygen environments. The new research indicates that the protein complexes of the transcription factor Ume6 manage the expression of these gene sets.
The recent discoveries concentrate on the Ume6 protein, which could be likened to a hitchhiker that interacts with other proteins to achieve its purpose.
“Consider microbial biofilms … like kitchen wrap that enables a community of cells to adhere together and frequently attach to a surface.”
—Aaron Mitchell, Franklin College of Arts and Sciences
“Visualize microbial biofilms as less of a cinematic experience and more akin to kitchen wrap that allows a group of cells to bond and typically stick to a surface, such as an implanted device,” Mitchell noted.
“Our discoveries clarify why Ume6 is so proficient at promoting biofilm and hypha formation: Its direct targets encompass numerous genes acknowledged as essential for these processes.”
Published in Nature Microbiology, this study was co-authored by UGA’s Eunsoo Do and Katharina Goerlich; Carnegie Mellon’s Joel McManus; Manning Y. Huang, previously at Carnegie Mellon and now at the University of California San Francisco; along with Robert Zarnowski and David R. Andes from the University of Wisconsin, Madison.
The post Life-saving devices may also provide the ideal setting for lethal fungal infections appeared first on UGA Today.