“`html

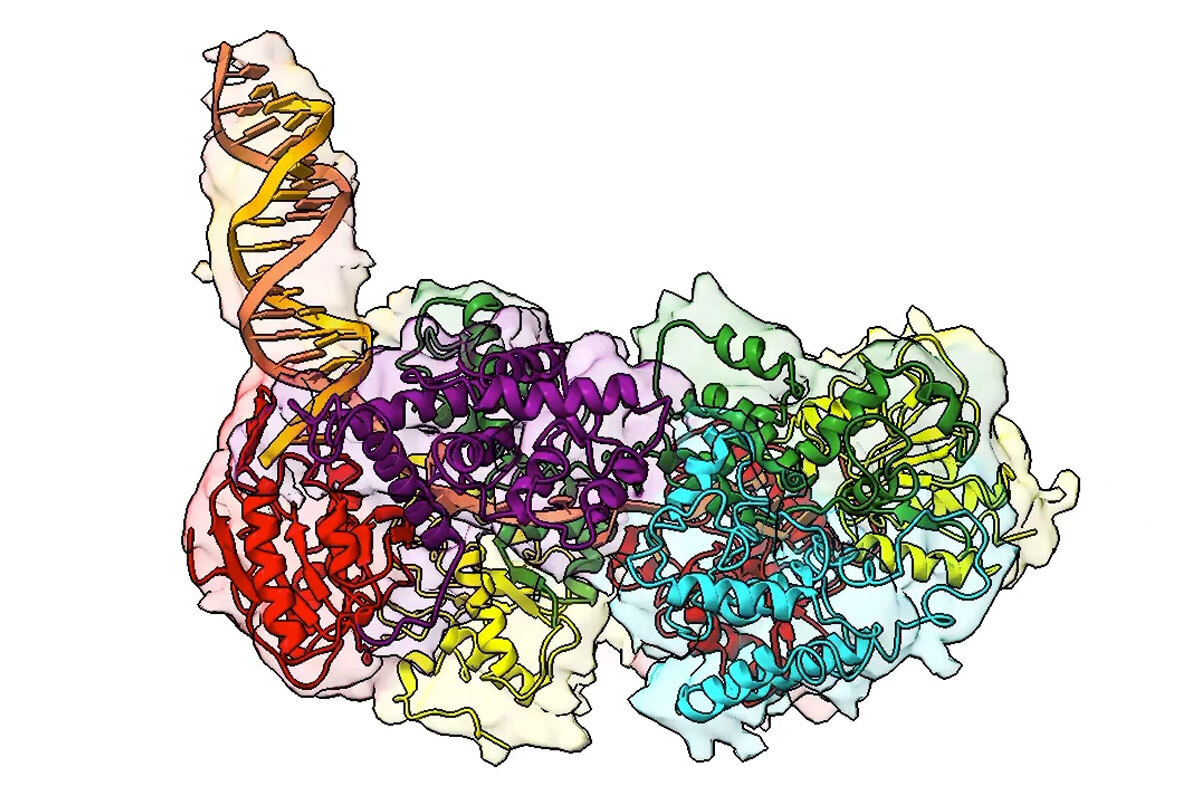
Scientists at Washington University School of Medicine in St. Louis have disclosed the configuration of an essential protein that plays a role in unwinding DNA for repair processes. Since this protein is vital for DNA repair in various organisms, including those that are responsible for diseases such as M. tuberculosis and E. coli, comprehending its function might pave the way for developing novel strategies to inhibit pathogen proliferation.
For an extended period, biochemical evidence indicated that this protein — a helicase enzyme named UvrD1 — needs to be formed from two smaller components to effectively unzip the DNA strand. However, structural assessments consistently only identified a singular complex, referred to as a monomer. Presently, a new investigation published in the Proceedings of the National Academy of Sciences unveils the first two-part formation — termed as a dimer — of this significant helicase enzyme, addressing questions pertaining to the 3D structure and regulation of the enzyme activity that have perplexed the discipline for decades.
The research was spearheaded by Eric Galburt, a biochemistry and molecular biophysics professor; and Ankita Chadda, who carried out this work as a graduate scholar in the Galburt lab and is currently a postdoctoral researcher at the Salk Institute for Biological Studies in San Diego; together with Timothy Lohman, the Marvin A. Brennecke Professor of Biophysics, and Binh Nguyen, a staff scientist in the Lohman lab.
Their findings also propose potential methods to disrupt the UvrD1 enzyme, which could be beneficial in formulating new therapeutics for tuberculosis or other ailments caused by organisms that depend on these helicases for DNA repair.
The post Key component to cell division unveiled in 3D appeared first on The Source.
“`