“`html
Cancer cells possess a singular, insatiable aim: to proliferate and replicate. While the majority remain clustered within the primary tumor, some rebellious cells detach to journey to far-off organs. In those locations, they can remain inactive — unseen and not multiplying — for extended periods, resembling hidden explosives poised to detonate.
This movement of cancer cells, known as metastasis, is particularly prevalent in breast cancer. For numerous patients, the illness may resurface months — or even years — after the initial treatment, this time manifesting in a completely different organ.
Robert Weinberg, the Daniel K. Ludwig Professor for Cancer Research at MIT and a founding member of the Whitehead Institute for Biomedical Research, has dedicated decades to deciphering the intricate biology of metastasis and advancing research that could enhance survival rates among individuals with metastatic breast cancer — or altogether prevent metastasis.
In his latest research, Weinberg, postdoc Jingwei Zhang, and their team pose a vital inquiry: What initiates these inactive cancer cells to burst forth into intense growth and replication? The group’s results, published on September 1 in The Proceedings of the National Academy of Sciences (PNAS), highlight an unusual instigator.
This awakening of dormant cancer cells, they have found, is not an arbitrary occurrence. Instead, the catalyst arises from the inflamed tissue surrounding the cells. One such instigator for this inflammation is bleomycin, a widely used chemotherapy drug known to scar and thicken lung tissue.
“The inflammation startles the dormant cancer cells into action,” Weinberg states. “Once stirred, they begin to multiply once more, initiating new, potentially fatal tumors in the body.”
Deciphering metastasis
There remains a significant amount that scientists have yet to uncover about metastasis, but this much is evident: Cancer cells must undertake a long and challenging expedition to accomplish it. The initial step is to detach from their nearby neighbors within the original tumor.
Typically, cells adhere to one another using surface proteins that function as molecular “velcro,” yet certain cancer cells can develop genetic alterations that disrupt the synthesis of these proteins, rendering them more agile and invasive, enabling them to dislodge from the parent tumor.
Once they have disconnected, they can invade blood vessels and lymphatic channels, which serve as thoroughfares to distant organs.
While many cancer cells succumb at some juncture during this journey, a handful survive. These cells exit the bloodstream and infiltrate various tissues—lungs, liver, bone, and even the brain — giving rise to new, frequently more aggressive tumors.
“Nearly 90 percent of cancer-related fatalities arise not from the initial tumor, but when cancer cells disseminate to other areas of the body,” remarks Weinberg. “This is why it’s crucial to comprehend how these ‘sleeping’ cancer cells can awaken and begin to proliferate again.”
Establishing residence in new tissue involves shifts in the environment — the “tumor microenvironment” — which the cancer cells may not adapt to well. These cells encounter continual dangers, including detection and assault by the immune system.
To endure, they often enter a protective phase of dormancy that halts growth and division. This dormant condition also renders them resistant to standard cancer therapies, which typically target rapidly dividing cells.
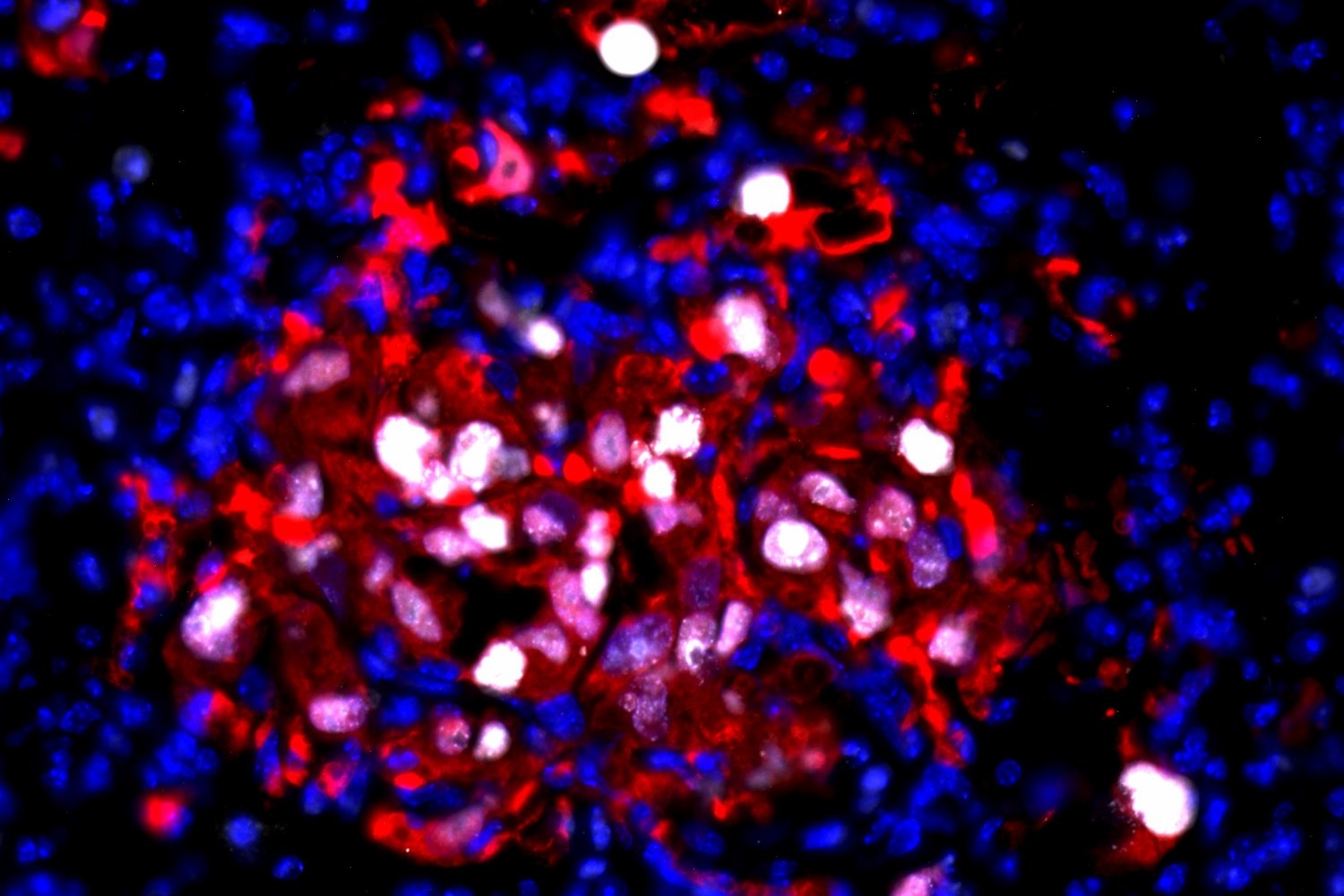
To explore what renders this dormancy reversible months or years later, researchers in the Weinberg Lab administered human breast cancer cells into mice. These cancer cells were altered to produce a fluorescent protein, allowing scientists to monitor their behavior within the host.
The team then concentrated on cancer cells that had embedded themselves in the lung tissue. By assessing these cells for particular proteins — Ki67, ITGB4, and p63 — serving as indicators of cell activity and condition, the researchers confirmed that these cells were in a non-dividing, dormant state.
Past research from the Weinberg Lab had established that inflammation in organ tissue could stimulate dormant breast cancer cells to restart growth. In this investigation, the team examined bleomycin — a chemotherapy drug recognized for inducing lung inflammation — which can be administered to patients post-surgery to diminish the risk of cancer reappearance.
The researchers discovered that lung inflammation induced by bleomycin was adequate to incite the formation of large lung cancer colonies in treated mice — and to transform the characteristics of these once-inactive cells into those that are more invasive and mobile.
Focusing on the tumor microenvironment, the team identified a type of immune cell known as M2 macrophages as catalysts of this phenomenon. These macrophages secrete molecules termed epidermal growth factor receptor (EGFR) ligands, which attach to receptors on the surface of dormant cancer cells. This initiates a cascade of signaling that compels dormant cancer cells to commence rapid multiplication.
However, EGFR signaling serves only as the initial ignition that sparks the blaze. “We discovered that once dormant cancer cells are roused, they preserve what we refer to as an ‘awakening memory,’” Zhang notes. “They no longer need continuous inflammatory signals from the microenvironment to remain active [growing and multiplying] — they recall the awakened state.”
Although signals related to inflammation are essential to awaken dormant cancer cells, the precise amount of signaling required remains uncertain. “This facet of cancer biology is particularly intricate, as multiple signals contribute to the transformation in these dormant cells,” Zhang remarks.
The team has already pinpointed one significant factor in the awakening process, yet comprehending the complete array of signals and how each influences the process is considerably more complicated — a question they continue to explore in their ongoing research.
Examining these critical transitions in the lives of cancer cells — such as their shift from dormancy to active proliferation — will enhance our scientific comprehension of metastasis and, as researchers in the Weinberg Lab aspire, lead to more effective treatments for individuals with metastatic cancers.
“`