Research from an increasing number of laboratories has revealed neurological advantages from subjecting human participants or animal models to light, sound, and/or tactile stimuli at the brain’s “gamma” frequency rhythm of 40Hz. In the most recent investigation at The Picower Institute for Learning and Memory and Alana Down Syndrome Center at MIT, researchers discovered that 40Hz sensory stimulation enhanced cognitive functions, circuit connectivity, and fostered the creation of new neurons in mice genetically modified to represent Down syndrome.
Li-Huei Tsai, Picower Professor at MIT and lead author of the recent study in PLOS ONE, states that while the findings are promising, further effort is required to evaluate if the technique, termed GENUS (gamma entrainment using sensory stimulation), might yield clinical advantages for individuals with Down syndrome. Her team has initiated a small-scale study involving human participants at MIT.
“While this investigation, for the first time, demonstrates beneficial impacts of GENUS on Down syndrome utilizing an imperfect mouse model, we must proceed with caution, as data confirming efficacy in humans is still lacking,” Tsai remarked, who oversees The Picower Institute and The Alana Center, and is part of MIT’s Department of Brain and Cognitive Sciences faculty.
Nonetheless, she emphasizes that the freshly published paper contributes evidence that GENUS can elicit a comprehensive, restorative, “homeostatic” health reaction in the brain amidst various pathologies. Most GENUS studies have focused on Alzheimer’s disease in humans or mice, but others have observed positive effects from the stimulation for ailments such as “chemo brain” and stroke.
Benefits for Down syndrome
In the research, the team led by postdoc Md Rezaul Islam and Brennan Jackson PhD ’23 utilized the “Ts65Dn” mouse model commonly associated with Down syndrome. This model encapsulates key features of the condition, although it does not perfectly replicate the human scenario, which is triggered by an additional copy of chromosome 21.
In the initial series of experiments detailed in the paper, the team demonstrated that an hour of daily exposure to 40Hz light and sound for three weeks correlated with notable enhancements on three standard short-term memory evaluations — two involving novel versus familiar recognition and one involving spatial navigation. Since these types of memory tasks engage a brain area termed the hippocampus, the researchers examined neural activity there and noted a significant rise in activity markers among mice who received GENUS stimulation compared to those who did not.
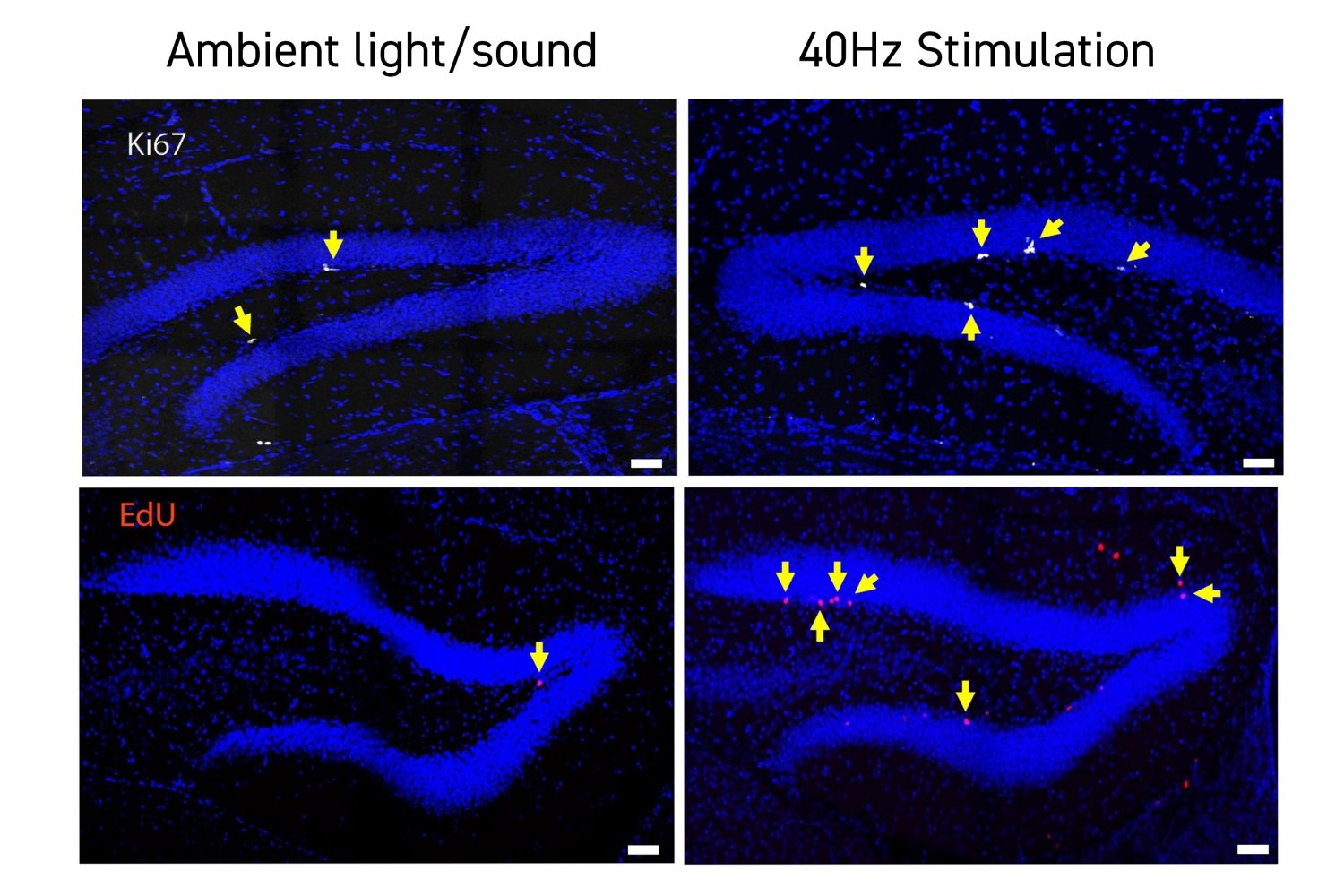
To gain deeper insight into how stimulated mice exhibited improved cognition, the researchers investigated whether cells in the hippocampus altered their gene expression patterns. They employed a technique known as single cell RNA sequencing, which provided data on how nearly 16,000 individual neurons and other cells transcribed their DNA into RNA, a vital step in gene expression. Many of the genes whose expression varied significantly between the stimulated and unstimulated mice were directly connected to forming and organizing neural circuit connections called synapses.
To validate the importance of this finding, the researchers directly inspected the hippocampus in both stimulated and control mice. They discovered that in a crucial subregion, the dentate gyrus, stimulated mice possessed a markedly greater number of synapses.
Delving deeper
The team not only analyzed gene expression of individual cells but also evaluated the data for patterns of coordination across numerous genes. They indeed identified several such “modules” of co-expression. Some of this evidence further supported the notion that 40Hz-stimulated mice made significant strides in synaptic connectivity, while another pivotal discovery pointed to a role for TCF4, an essential regulator of gene transcription necessary for generating new neurons, or “neurogenesis.”
The team’s genetic analysis indicated that TCF4 is underexpressed in Down syndrome mice, yet the researchers observed enhanced TCF4 expression in GENUS-stimulated mice. When the team proceeded to the lab bench to assess whether the mice displayed differences in neurogenesis, they found direct evidence that stimulated mice exhibited greater neurogenesis in the dentate gyrus compared to their unstimulated counterparts. While these increases in TCF4 expression and neurogenesis are correlational, the researchers hypothesize that the rise in new neurons likely elucidates at least a portion of the enhancements in new synapses and improved short-term memory capabilities.
“The heightened presumed functional synapses in the dentate gyrus are likely linked to the increased adult neurogenesis observed in Down syndrome mice following GENUS treatment,” remarks Islam.
This study marks the first documentation that GENUS is associated with enhanced neurogenesis.
The analysis of gene expression modules also unveiled other critical insights. One such finding revealed that a cluster of genes, which typically shows decreased expression with normal aging and in Alzheimer’s disease, maintained higher expression levels among mice who underwent 40Hz sensory stimulation.
Furthermore, the researchers indicated that stimulated mice retained a higher number of Reelin-expressing cells in the hippocampus. Neurons expressing Reelin are particularly susceptible in Alzheimer’s disease; however, the presence of this protein is associated with cognitive resilience amid Alzheimer’s disease pathology, which Ts65Dn mice develop. Approximately 90 percent of individuals with Down syndrome develop Alzheimer’s disease, usually post-40 years of age.
“In this study, we demonstrated that GENUS amplifies the proportion of Reln+ neurons in the hippocampus of a Down syndrome mouse model, implying that GENUS may enhance cognitive resilience,” remarks Islam.
Collectively with other studies, Tsai and Islam assert that the new findings bolster the argument that GENUS stimulates the brain at both the cellular and molecular levels to initiate a homeostatic response to irregularities induced by disease pathology, be it neurodegeneration in Alzheimer’s, demyelination in chemo brain, or deficits in neurogenesis in Down syndrome.
However, the authors also cautioned that the study had certain limitations. Not only is the Ts65Dn model an imperfect representation of human Down syndrome, but the mice utilized were exclusively male. Additionally, the cognitive evaluations conducted in the study solely focused on short-term memory. Finally, while the study was groundbreaking in its extensive examination of gene expression in the hippocampus amid GENUS stimulation, it did not explore alterations in other cognitively critical brain regions, such as the prefrontal cortex.
Alongside Jackson, Islam, and Tsai, the paper also includes contributions from Maeesha Tasnim Naomi, Brooke Schatz, Noah Tan, Mitchell Murdock, Dong Shin Park, Daniela Rodrigues Amorim, Fred Jiang, S. Sebastian Pineda, Chinnakkaruppan Adaikkan, Vanesa Fernandez, Ute Geigenmuller, Rosalind Mott Firenze, Manolis Kellis, and Ed Boyden.
Funding for the investigation came from the Alana Down Syndrome Center at MIT and the Alana USA Foundation, the U.S. National Science Foundation, the La Caixa Banking Foundation, a European Molecular Biology Organization long-term postdoctoral fellowship, Barbara J. Weedon, Henry E. Singleton, and the Hubolow family.