“`html
Prior to cell division, cells must first duplicate all of their chromosomes, ensuring that each of the resulting daughter cells acquires a complete set of genetic information. Up until now, researchers had assumed that during division, the genome loses its characteristic 3D internal configuration that it generally acquires.
Once division concludes, it was believed that the genome slowly recovers that intricate, globular form, which is crucial for regulating which genes are activated in a particular cell.
Nonetheless, a recent study from MIT indicates that this understanding is not entirely precise. Utilizing a higher-resolution genome mapping method, the research group found that small 3D loops linking regulatory elements and genes remain in the genome during cell division, or mitosis.
“This investigation significantly clarifies how we should conceptualize mitosis. Previously, mitosis was viewed as a blank canvas, devoid of transcription and any structure related to gene function. Now we understand that’s not entirely accurate,” states Anders Sejr Hansen, an associate professor of biological engineering at MIT. “What we observe is that structure is always present. It never disappears.”
The researchers further found that these regulatory loops seem to strengthen as chromosomes compact in preparation for cell division. This compaction brings genetic regulatory elements into closer proximity, prompting them to adhere together. This may aid cells in “remembering” interactions from one cell cycle to the subsequent one.
“The results connect the genome’s architecture to its role in managing gene activation and deactivation, which has been a major hurdle in the field for years,” mentions Viraat Goel PhD ’25, the lead author of the research.
Hansen and Edward Banigan, a research scientist at MIT’s Institute for Medical Engineering and Science, are the senior authors of the paper, which is published today in Nature Structural and Molecular Biology. Leonid Mirny, a professor in MIT’s Institute for Medical Engineering and Science and the Department of Physics, along with Gerd Blobel, a professor at the Perelman School of Medicine at the University of Pennsylvania, are also contributors to the study.
An unexpected revelation
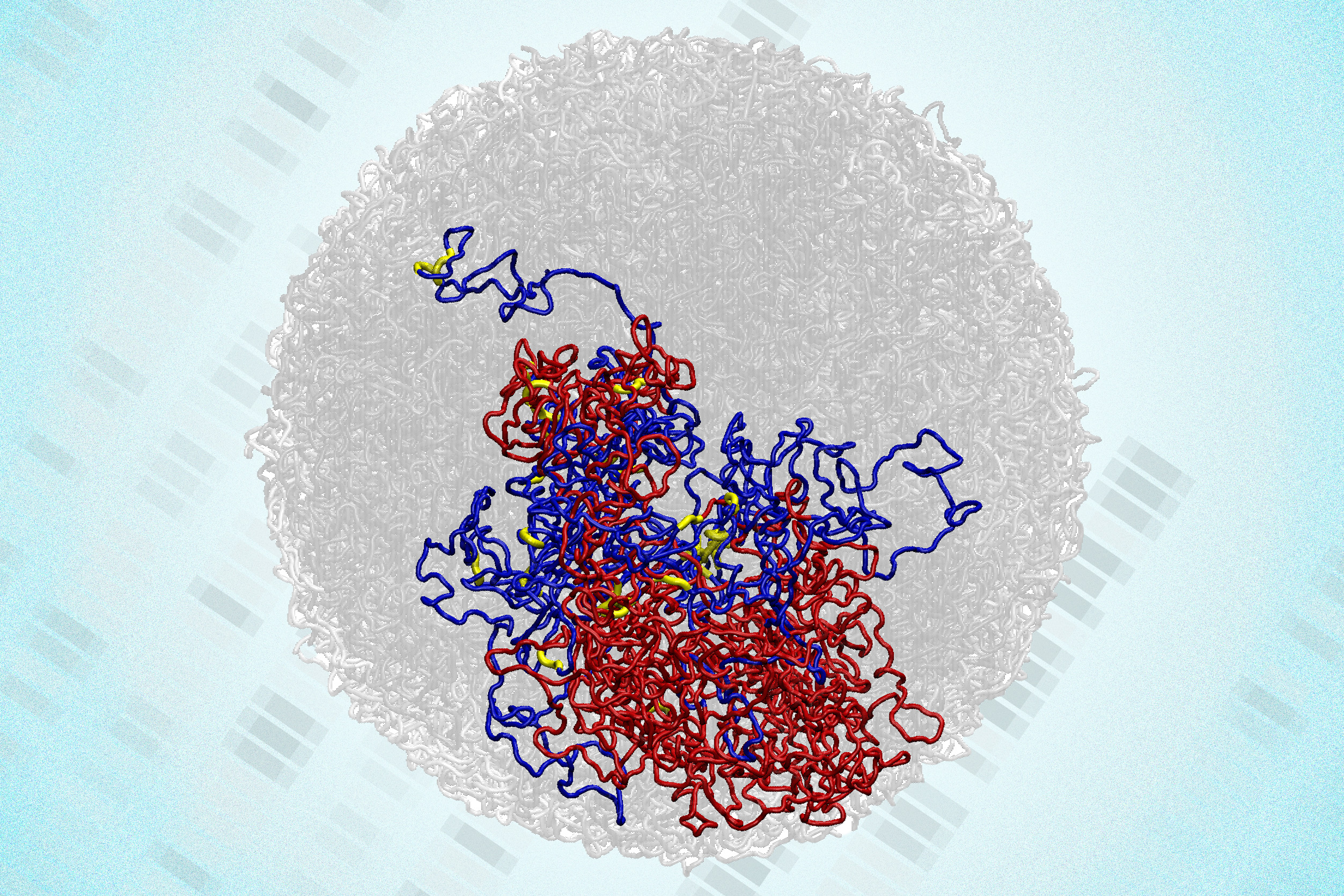
Over the last two decades, researchers have shown that within the cell nucleus, DNA arranges itself into 3D loops. While many loops facilitate interactions between genes and regulatory regions that may be separated by millions of base pairs, others form during cell division to condense chromosomes. Much of the mapping of these 3D structures has utilized a method known as Hi-C, originally developed by a team including MIT researchers under the leadership of Job Dekker at the University of Massachusetts Chan Medical School. To conduct Hi-C, researchers use enzymes to cut the genome into numerous small sections and biochemically link segments that are adjacent in 3D space within the cell nucleus. They then identify the interacting segments through sequencing.
However, this method lacks sufficient resolution to pinpoint all specific interactions between genes and regulatory elements, such as enhancers. Enhancers are short sequences of DNA that can aid in activating a gene’s transcription by binding to the gene’s promoter — the site where transcription initiates.
In 2023, Hansen and colleagues devised a novel methodology that enables them to examine 3D genome structures at 100 to 1,000 times the resolution previously achievable. This technique, termed Region-Capture Micro-C (RC-MC), employs a different enzyme that cleaves the genome into small fragments of similar length. It also concentrates on a smaller portion of the genome, facilitating high-resolution 3D mapping of a specific genomic region.
With this technique, the researchers were able to recognize a new type of genome structure previously unseen, which they named “microcompartments.” These are tiny, highly interconnected loops that arise when enhancers and promoters situated close to one another adhere together.
In that publication, experiments demonstrated that these loops were not generated by the same processes that create other genome structures, yet the researchers could not clarify how they do form. To address this question, the team set out to investigate cells during cell division. During mitosis, chromosomes condense significantly to enable replication, sorting, and distribution between two daughter cells. As this occurs, larger genomic structures like A/B compartments and topologically associating domains (TADs) vanish completely.
The researchers speculated that the microcompartments they had identified would also vanish during mitosis. By monitoring cells throughout the entire division process, they aimed to discern how the microcompartments emerge post-mitosis.
“It has been assumed that during mitosis, almost all gene transcription is halted. Additionally, prior to our publication, it was considered that all 3D structures associated with gene regulation were lost and replaced by compaction. It’s perceived as a complete reset every cell cycle,” Hansen explains.
Yet, to their astonishment, the researchers observed that microcompartments remained visible during mitosis, and in fact, they became more pronounced as the cell progressed through division.
“We approached this study with the expectation that, for certain, there would be no regulatory structure in mitosis, and then we unexpectedly discovered structure in mitosis,” Hansen reveals.
Employing their technique, the researchers also confirmed that larger structures like A/B compartments and TADs do dissipate during mitosis, as previously noted.
“This study harnesses the extraordinary genomic resolution of the RC-MC assay to unveil new and surprising facets of mitotic chromatin organization, which we have overlooked in the past using conventional 3C-based assays. The authors reveal that, contrary to the well-documented drastic loss of TADs and compartmentalization during mitosis, fine-scale ‘microcompartments’ — nested interactions between active regulatory elements — are preserved or even momentarily enhanced,” states Effie Apostolou, an associate professor of molecular biology in medicine at Weill Cornell Medicine, who was not involved in the research.
A rise in transcription
The discoveries may elucidate a surge in gene transcription that typically occurs near the conclusion of mitosis, according to the researchers. Since the 1960s, it was believed that transcription entirely ceased during mitosis, but studies in 2016 and 2017 demonstrated that cells experience a brief peak of transcription, which is quickly suppressed until the cell completes division.
In their recent study, the MIT team observed that during mitosis, microcompartments are more likely situated near the genes that experience a spike during cell division. They also found that these loops seem to form as a consequence of the genome compaction occurring during mitosis. This compaction draws enhancers and promoters nearer, enabling them to adhere and create microcompartments.
Once established, the loops that form microcompartments might inadvertently activate gene transcription, which is then repressed by the cell. Upon completion of division and entering a state referred to as G1, many of these small loops weaken or vanish.
“It almost appears that this transcriptional peak during mitosis is an unintended consequence of creating an unusually favorable environment for microcompartments to establish during mitosis,” asserts Hansen. “Then, the cell quickly prunes and discards many of those loops as it transitions into G1.”
As chromosome compaction can also be affected by a cell’s size and shape, the researchers are now investigating how variations in those characteristics influence genome structure and, in turn, gene regulation.
“We are considering some natural biological conditions where cells alter their shape and size, and whether we can potentially elucidate some 3D genome changes that previously lacked explanation,” Hansen notes. “Another critical question is how does the cell decide which microcompartments to retain and which to eliminate when transitioning into G1, to ensure the accuracy of gene expression?”
This research was partially supported by the National Institutes of Health, a National Science Foundation CAREER Award, the Gene Regulation Observatory of the Broad Institute, a Pew-Stewart Scholar Award for Cancer Research, the Mathers Foundation, the MIT Westaway Fund, the Bridge Project of the Koch Institute and Dana-Farber/Harvard Cancer Center, and the Koch Institute Support (core) Grant from the National Cancer Institute.
“`