“`html
The irritation, redness, and swelling of an allergic response are instigated by mast cells — the alert first responders of the immune system that react swiftly with histamine-laden granules when a perceived danger arises.
Currently, scientists at Washington University School of Medicine in St. Louis have unveiled that the same cells that bring distress to countless allergy sufferers can also defend the brain against bacterial and viral invasions. In mouse models, they observed that these mast cells vigilantly monitor small passages through which fluid waste exits the brain, launching a defense mechanism to seal the openings upon detecting a pathogen, thus thwarting the invaders from entering the brain.
The research was released on July 24 in Cell and may carry significant consequences for the prevention or treatment of brain infections.
“These discoveries open a completely new pathway for creating interventions that could safeguard the brain from infections,” stated senior author Jonathan Kipnis, PhD, the Alan A. and Edith L. Wolff Distinguished Professor of Pathology & Immunology at WashU Medicine and a BJC Investigator. “We now comprehend how mast cells protect the brain, allowing us to investigate enhancing their functionality amid infection threats.”
Such infectious conditions encompass bacterial meningitis, a potentially serious illness that affects the layers of tissue, known as meninges, that encase the brain beneath the skull. Small openings within these layers form pathways that transport fluid waste away from the brain into lymphatic vessels, where immune cells surveil the fluid for indicators of danger or infection. However, these entrances can also offer avenues for bacteria to invade.
Kipnis’ laboratory had identified the existence of lymphatic vessels in the mouse dura mater, the outermost tissue layer surrounding the brain beneath the skull, where brain fluid flows through small openings. To further elucidate how such fluid movement is coordinated, Kipnis’ lab teamed up with Felipe Almeida de Pinho Ribeiro, PhD, an assistant professor of medicine at WashU Medicine who explores neuroimmune interactions contributing to human ailments. Alongside Tornike Mamuladze, MD, an immunology graduate student in Kipnis’ lab, they discovered that mice afflicted with Streptococcus agalactiae or S. pneumoniae, strains of round bacteria responsible for meningitis, exhibited diminished brain fluid flow through the openings compared to healthy mice.
They found that the presence of bacteria in the tissues surrounding the mouse brain triggered mast cells to release histamine-containing granules, which caused the veins passing through the tiny openings to expand. By swelling into the area where brain fluid typically flows, the dilated veins formed a temporary barrier that also impeded bacteria from entering and infecting the brain.
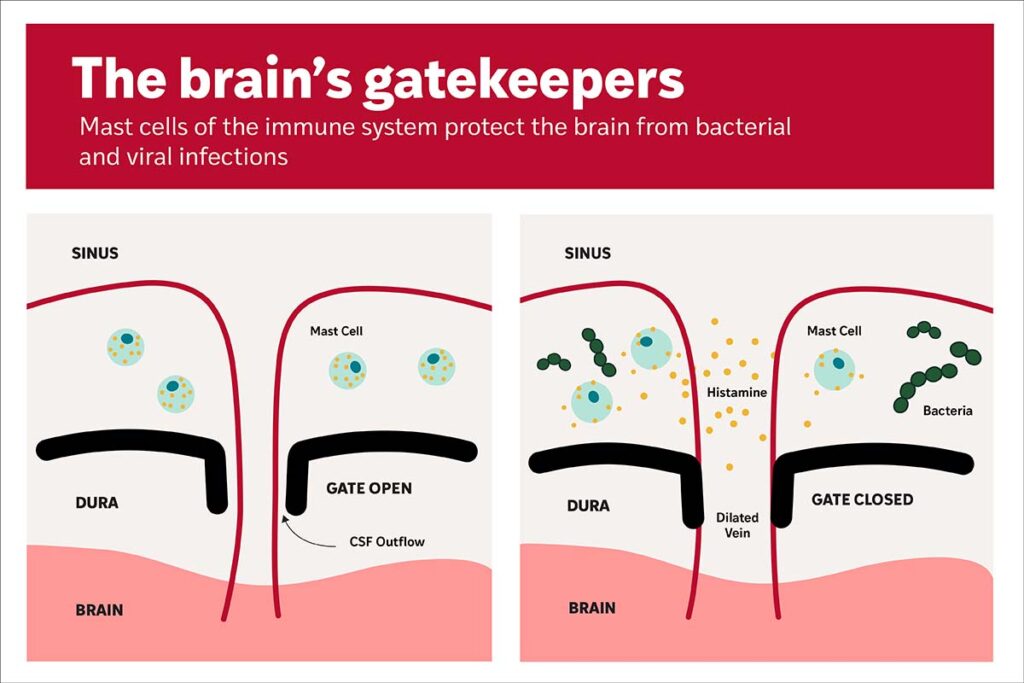
The research discovered that activated mast cells also trigger a swift immune response, attracting bacteria-consuming immune cells known as neutrophils to eliminate the pathogens in the infected tissue. When mice in the study were devoid of mast cells, a greater number of bacteria penetrated the brain via the small openings, whereas enhancing mast cell activity prior to infection lowered the bacterial presence.
To investigate if viral invaders were similarly obstructed by mast cells, the team collaborated with Michael S. Diamond, MD, PhD, the Herbert S. Gasser Professor of Medicine at WashU Medicine, who is internationally acknowledged for his research on understanding how Zika, West Nile, chikungunya, and other emerging viruses interact with and evade the body’s immune defenses. The researchers also identified an increased presence of the West Nile virus, which is transmitted through the bite of an infected mosquito, in the brains of infected mice lacking mast cells as opposed to those with mast cells.
“We believe that enhancing the function of mast cells could aid in safeguarding the brain from bacterial and viral infections,” remarked Kipnis. “However, the activation of mast cells is a double-edged sword. Prolonged activation of these cells hampers fluid movement and could potentially lead to the accumulation of waste, like amyloid beta, in the brain.”
In upcoming research, the team plans to explore whether chronic activation of mast cells could have detrimental effects on Alzheimer’s disease, which is marked by an accumulation of amyloid beta.
“Mast cells play a significant role in brain health,” stated Mamuladze, the primary author of the research. “Understanding how to manipulate their function at the gateways to the brain to block pathogens while allowing waste to exit will be vital for promoting brain health.”
Mamuladze T, Zaninelli TH, Smyth LCD, Wu Y, Abramishvili D, Silva R, Imbiakha B, Verhaege D, Du S, Papadopoulos Z, Gu X, Lee D, Storck S, Perrin RJ, Smirnov I, Dong X, Song Hu, Diamond MS, Pinho-Ribeiro FA, Kipnis J. Mast cells regulate the brain-dura interface and CSF dynamics. Cell. July 24, 2025. DOI: 10.1016/j.cell.2025.06.046.
This research was supported by grants from the National Institutes of Health, grant numbers R01AI183879, P01AG078106, and R37AG034113, as well as the Cure Alzheimer’s Fund BEE consortium. The content solely reflects the authors’ views and does not necessarily represent the official perspectives of the NIH.
Kipnis is a co-founder of Rho Bio. Diamond serves as a consultant or advisor for Inbios, Moderna, IntegerBio, Merck, GlaxoSmithKline, Bavarian Nordic, and Akagera Medicines. The Diamond laboratory has received unrelated funding support through sponsored research agreements from Emergent BioSolutions, Bavarian Nordic, Moderna, and IntegerBio.
About Washington University School of Medicine
WashU Medicine stands as a prominent leader in academic medicine, encompassing biomedical research, patient care, and educational initiatives with 2,900 faculty members. Its National Institutes of Health (NIH) research funding portfolio is the second largest among U.S. medical schools, having grown by 83% since 2016. Along with institutional investments, WashU Medicine dedicates over $1 billion each year to fundamental and clinical research innovation and education. Its faculty practice consistently ranks among the top five in the nation, employing over 1,900 faculty physicians across 130 locations. WashU Medicine physicians solely staff Barnes-Jewish and St. Louis Children’s hospitals — the academic facilities of BJC HealthCare — and provide care at BJC’s community hospitals within the region. With a notable history in MD/PhD training, WashU recently allocated $100 million towards scholarships and renewing its medical school curriculum, and it hosts outstanding training programs in various medical subspecialties as well as physical therapy, occupational therapy, and audiology and communication sciences.
Originally published on the WashU Medicine website
The article Immune ‘bouncers’ protect the brain from infection first appeared on The Source.
“`