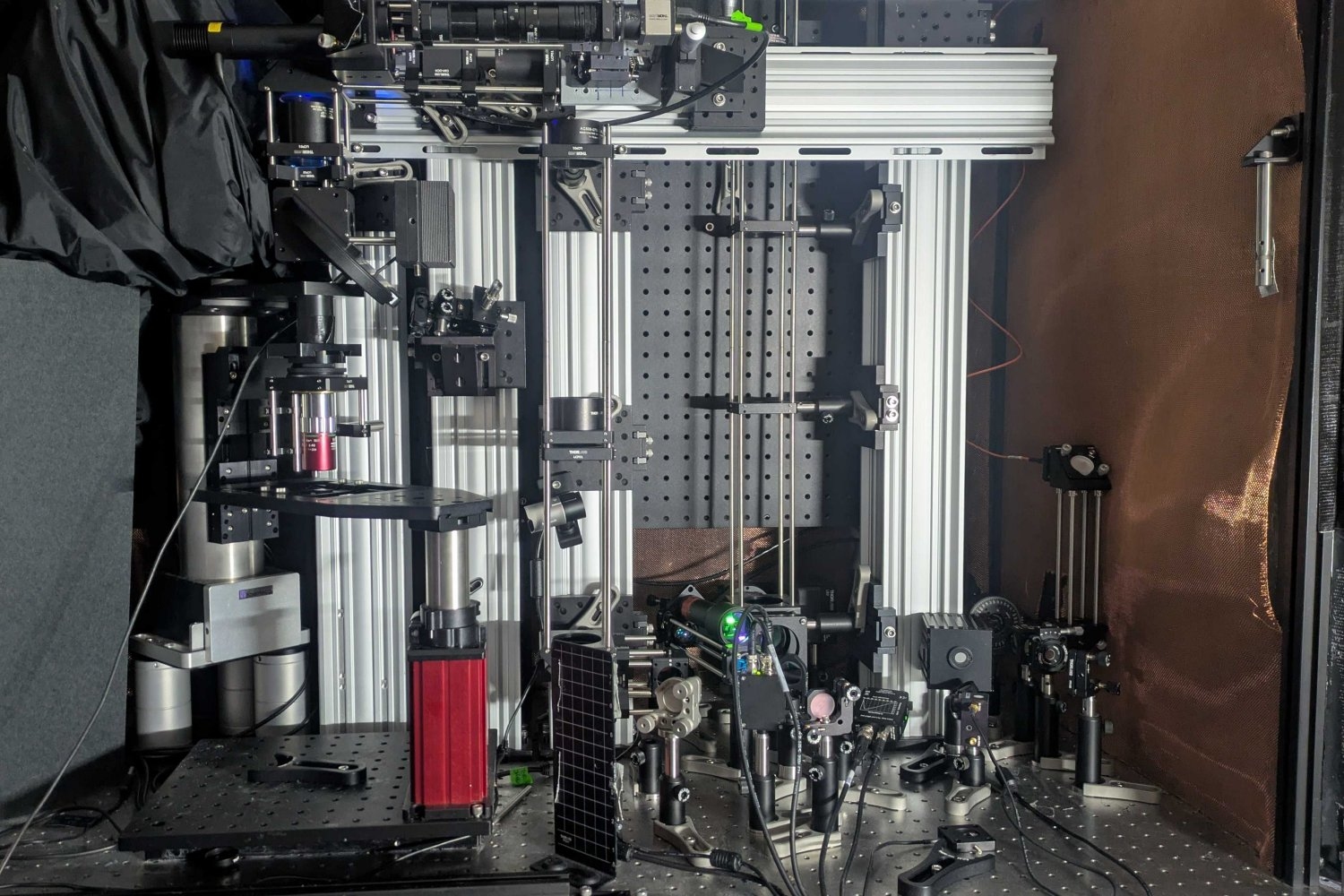
For both scientific inquiry and medical applications, scholars have invested years advancing microscopy techniques to yield progressively profound and clearer images of brain function, not only within the cortex but also in underlying areas such as the hippocampus. In a recent investigation, a group of scientists and engineers at MIT unveils a novel microscope system that can exceptionally delve deep into brain tissues to observe the molecular activities of individual cells using sound.
“The principal breakthrough here is allowing us to visualize deeper at single-cell clarity,” states neuroscientist Mriganka Sur, a lead author alongside mechanical engineering professor Peter So and principal research scientist Brian Anthony. Sur holds the Paul and Lilah Newton Professorship at The Picower Institute for Learning and Memory and the Department of Brain and Cognitive Sciences at MIT.
In the journal Light: Science and Applications, the team illustrates their ability to identify NAD(P)H, a molecule closely related to cellular metabolism in general and neuronal electrical activity in particular, throughout samples such as a 1.1-millimeter “cerebral organoid,” a 3D miniature brain-like tissue generated from human stem cells, and a 0.7-milimeter-thick slice of mouse brain tissue.
In fact, co-lead author and mechanical engineering postdoctoral researcher W. David Lee, who envisioned the innovative design of the microscope, mentions that the system could have examined even deeper, but the sample sizes were not sufficient to demonstrate that capability.
“That’s when we encountered the glass on the opposite side,” he explains. “I believe we are quite assured about exploring greater depths.”
Nonetheless, a depth of 1.1 millimeters is more than five times deeper than other microscopy technologies can detect NAD(P)H within dense brain tissues. The new system achieved this depth and clarity by integrating several advanced techniques to precisely and effectively excite the molecule and subsequently detect the resultant energy, all without the need for adding any external labels, whether through chemicals or genetically engineered fluorescence.
Instead of concentrating the necessary NAD(P)H excitation energy on a neuron using near-ultraviolet light at its typical peak absorption, the microscope achieves excitation by focusing an intense, extremely brief burst of light (lasting a quadrillionth of a second) at three times the regular absorption wavelength. Such “three-photon” excitation penetrates extensively into tissue with reduced scattering due to the longer wavelength of light (“like fog lamps,” remarks Sur). Meanwhile, while the excitation generates a faint fluorescent light signal from NAD(P)H, the majority of absorbed energy causes a localized (approximately 10 microns) thermal expansion within the cell, generating sound waves that traverse tissue more easily than fluorescence emissions. A sensitive ultrasound microphone within the microscope captures these waves, and with sufficient sound data, software translates them into high-resolution images (similarly to a sonogram). This imaging method is termed “three-photon photoacoustic imaging.”
“We have combined all these techniques — three-photon, label-free, photoacoustic detection,” explains co-lead author Tatsuya Osaki, a research scientist in Sur’s lab at the Picower Institute. “We amalgamated all these innovative techniques into one streamlined process to create this ‘Multiphoton-In and Acoustic-Out’ platform.”
Lee and Osaki collaborated with research scientist Xiang Zhang and postdoctoral fellow Rebecca Zubajlo to spearhead the study, in which the team demonstrated consistent detection of the sound signal through the samples. So far, they have generated visual representations from the sound at various depths as they enhance their signal processing.
In the study, the team also presents concurrent “third-harmonic generation” imaging, which arises from the three-photon stimulation and provides a detailed rendering of cellular structures, alongside their photoacoustic imaging, identifying NAD(P)H. They also mention that their photoacoustic approach could identify additional molecules such as the genetically encoded calcium indicator GCaMP, utilized by neuroscientists to indicate neural electrical activity.
With the concept of label-free, multiphoton, photoacoustic microscopy (LF-MP-PAM) detailed in the paper, the team is now looking forward to both neuroscience and clinical applications.
For instance, through the company Precision Healing, Inc., which he established and later sold, Lee has already proven that NAD(P)H imaging can assist in wound healing. In the brain, levels of this molecule are known to fluctuate in conditions such as Alzheimer’s disease, Rett syndrome, and seizures, making it a potentially significant biomarker. Since the new system is label-free (i.e., no added chemicals or modified genes), it has potential applicability in human subjects, for instance, during brain surgeries.
The team’s subsequent step is to validate the system in live animals rather than merely in in vitro or ex vivo tissues. The technical hurdle is that the microphone cannot remain positioned on the opposite side of the sample from the light source (as it was in the current study). It needs to be positioned on top, alongside the light source.
Lee expresses optimism that full imaging at depths of 2 millimeters in living brains is entirely achievable, given the findings of this new study.
“In principle, it should function,” he asserts.
Mercedes Balcells and Elazer Edelman are also contributors to the paper. Financial support for the research was provided by various sources, including the National Institutes of Health, the Simon Center for the Social Brain, Peter So’s lab, The Picower Institute for Learning and Memory, and the Freedom Together Foundation.