“`html
Efficiently eliminating carbon dioxide from the atmosphere is frequently regarded as a vital necessity for addressing climate change, yet methods for extracting carbon dioxide face a compromise. Chemical substances that effectively capture CO₂ from the air do not readily release it once it has been trapped, while substances that release CO₂ efficiently are not particularly adept at capturing it. Enhancing one aspect of the cycle typically leads to a decline in the other aspect.
Now, utilizing nanoscale filtration membranes, researchers at MIT have implemented a straightforward intermediate step that aids both phases of the cycle. The innovative method could enhance the effectiveness of electrochemical carbon dioxide capture and release by a factor of six and reduce expenses by at least 20 percent, they claim.
The latest discoveries are disclosed today in the journal ACS Energy Letters, in a paper authored by MIT doctoral candidates Simon Rufer, Tal Joseph, and Zara Aamer, alongside professor of mechanical engineering Kripa Varanasi.
“We need to consider scale from the outset when dealing with carbon capture, as making a significant impact necessitates processing gigatons of CO₂,” states Varanasi. “Adopting this perspective enables us to identify essential bottlenecks and devise creative solutions with tangible potential for impact. This drives our research.”
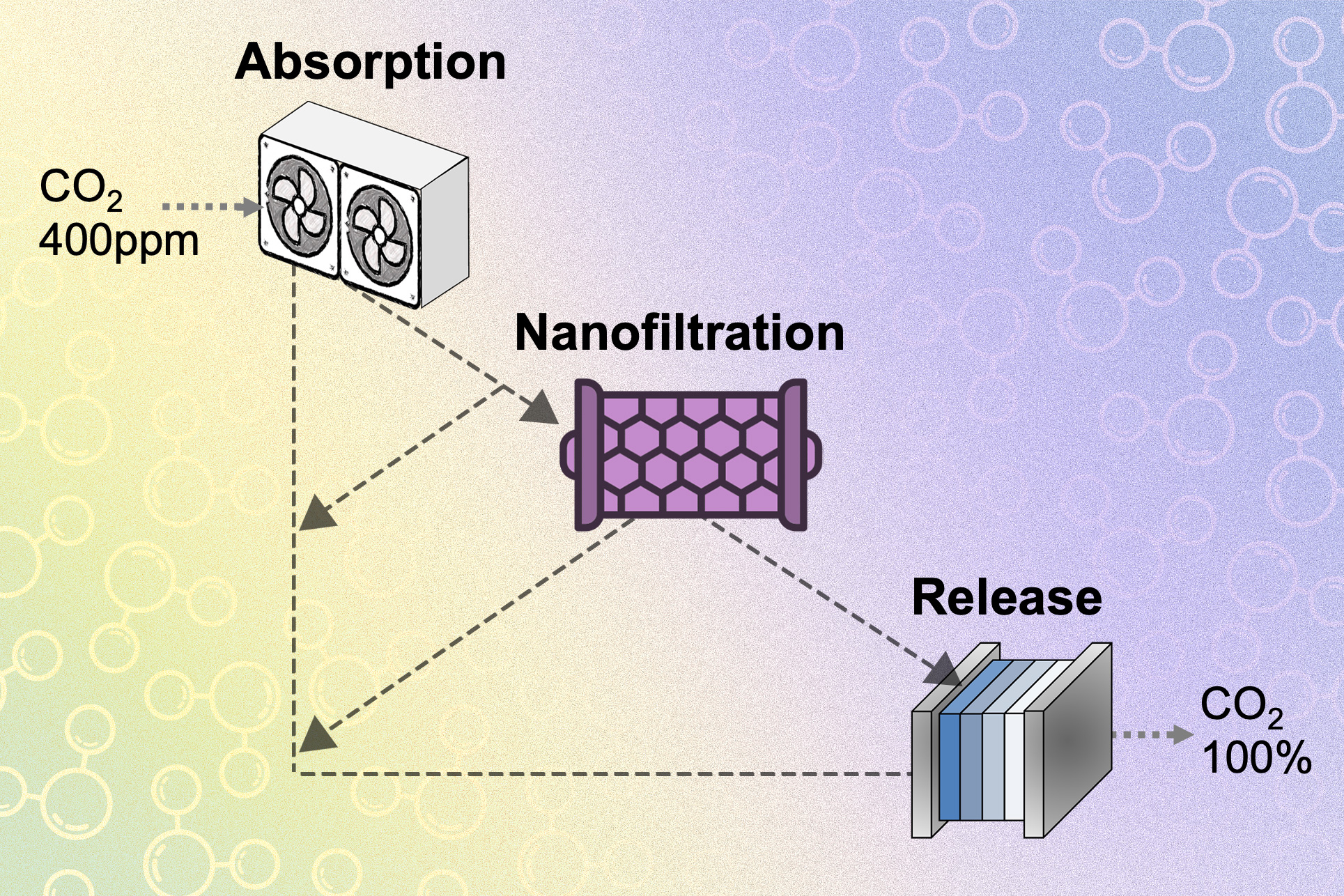
Numerous carbon-capture systems operate utilizing chemicals known as hydroxides, which readily react with carbon dioxide to create carbonate. This carbonate is then introduced into an electrochemical cell, where it interacts with an acid to produce water and liberate carbon dioxide. The procedure can take ordinary air containing approximately 400 parts per million of carbon dioxide and generate a flow of 100 percent pure carbon dioxide, which can subsequently be transformed into fuels or other products.
Both the capturing and releasing stages operate within the same water-based solution, although the first phase requires a liquid with a high concentration of hydroxide ions, while the second phase demands one rich in carbonate ions. “You can observe how these two phases conflict,” explains Varanasi. “Both systems circulate the same sorbent back and forth. They use the identical liquid. However, as they require two different types of solutions for optimal operation, it becomes unfeasible to run both systems at their most efficient settings.”
The solution devised by the team was to separate the two phases of the system and introduce a third phase in between. Essentially, after the hydroxide in the first phase has been primarily transformed into carbonate, specialized nanofiltration membranes distinctively separate ions in the solution according to their charge. Carbonate ions carry a charge of 2, while hydroxide ions carry a charge of 1. “The nanofiltration effectively distinguishes these two,” asserts Rufer.
After separation, the hydroxide ions are returned to the absorption side of the system, while the carbonates are directed to the electrochemical release phase. In this manner, both extremities of the system can function within their optimal efficiency ranges. Varanasi clarifies that in the electrochemical release stage, protons are introduced to the carbonate to facilitate the conversion into carbon dioxide and water; however, if hydroxide ions are also present, the protons will react with those ions instead, resulting in the mere production of water.
“If you don’t segregate these hydroxides and carbonates,” Rufer asserts, “the failure of the system occurs because you’ll add protons to hydroxide instead of carbonate, and thus you’ll merely generate water instead of extracting carbon dioxide. That’s where efficiency is compromised. Employing nanofiltration to circumvent this issue was something we hadn’t seen proposed before.”
Tests demonstrated that the nanofiltration could separate the carbonate from the hydroxide solution with around 95 percent efficiency, validating the idea under practical conditions, Rufer notes. The subsequent phase involved evaluating how significantly this would influence the overall efficiency and economics of the process. They developed a techno-economic model, accounting for electrochemical efficiency, voltage, absorption rate, capital costs, nanofiltration efficiency, and additional factors.
The analysis revealed that current systems incur expenses of at least $600 per ton of captured carbon dioxide, while incorporating the nanofiltration component reduces that to approximately $450 per ton. Moreover, the new system exhibits substantially greater stability, maintaining high efficiency even amid fluctuations in the ion concentrations within the solution. “In the previous system lacking nanofiltration, you’re essentially operating on a precarious edge,” Rufer remarks; if the concentration changes even slightly in either direction, efficiency drastically declines. “However, with our nanofiltration system, it effectively acts as a buffer, making it much more forgiving. You possess a far broader operational domain, enabling significantly lower costs.”
He mentions that this approach could be applied not only to the direct air capture systems they scrutinized but also to point-source systems — those directly linked to emission sources such as power plant discharges — or the next phase of the process, converting captured carbon dioxide into useful products like fuel or chemical feedstocks. Those conversion processes, he states, “also face bottlenecks in this carbonate and hydroxide trade-off.”
Furthermore, this technology could pave the way for safer alternative chemistries in carbon capture, Varanasi notes. “Many of these absorbents can occasionally be toxic or harmful to the environment. By implementing a system like ours, you can enhance the reaction rate, enabling the selection of chemistries that may not exhibit the best absorption rate initially but can be refined to ensure safety.”
Varanasi adds, “The truly commendable aspect of this is that we’ve managed to accomplish this utilizing commercially available materials,” and with a system that can be effortlessly retrofitted to existing carbon-capture facilities. If further cost reductions can be achieved down to about $200 per ton, it could render the system viable for widespread implementation. With ongoing efforts, he expresses confidence that “we’ll develop something that can become economically feasible” and ultimately generate valuable, marketable products.
Rufer observes that even at present, “individuals are purchasing carbon credits at a rate exceeding $500 per ton. Therefore, at the anticipated cost, it’s already commercially viable, as some buyers are prepared to pay that price.” However, by lowering the price even further, this should amplify the number of potential buyers interested in acquiring the credits, he suggests. “It’s merely a matter of how extensively we can scale it.” Recognizing this burgeoning market demand, Varanasi states, “Our objective is to deliver the industry scalable, cost-effective, and dependable technologies and systems that empower them to achieve their decarbonization goals.”
The research received backing from Shell International Exploration and Production Inc. through the MIT Energy Initiative and the U.S. National Science Foundation, while leveraging the facilities at MIT.nano.
“`