“`html
Science & Tech
Extremely attentive science

Veasey Conway/Harvard Staff Photographer
David Ginty explores delight and discomfort to illuminate autism and additional conditions
The irritation from a clothing tag. The seam on a sock’s interior. The tickling sensation of hair on your neck. For many individuals, these feelings can be easily disregarded as we go about our daily routines. Yet, for some autistic individuals, regular sensations can be unbearable.
David Ginty understands the reasons, and contrary to past beliefs among some autism researchers, it does not stem from a malfunction in the brain.
Ginty, the Edward R. and Anne G. Lefler Professor of Neurobiology and head of the Department of Neurobiology at Harvard Medical School, investigates sensations of touch and pain. Scientists have known for a while, according to him, that our perception of physical sensations results from cooperation between our brain, central nervous system, and sensory neurons. However, the specifics of this cooperation have stayed elusive, and finding answers holds significant ramifications for treating various issues, ranging from chronic pain to hypersensitivity in autism to sexual dysfunction.
“The auditory system is concerned with sound waves within a specific frequency range,” Ginty noted. “The visual system similarly only focuses on a narrow spectrum of visible light. Yet, the somatosensory system engages with tactile stimuli, thermal inputs, chemical signals, proprioception — understanding the position of our body and limbs in both space and time, along with the status of many of our bodily organs.
“Moreover, there exists an emotional aspect, an affective dimension of touch, which is itself a rapidly expanding field that is quite intriguing. How does touch provoke an emotional reaction? Somatosensation is profoundly intricate and multidimensional.”
“We’re diligently searching for non-opioid methods to address pain, and we’ve discovered numerous potential strategies.”
Approximately a decade ago, Ginty and his colleagues discovered that in animal models of autism spectrum disorder, the source of sensory dysfunction was not the brain, as was previously believed, but rather the spinal cord and peripheral system. The crucial components are second-order neurons in the spinal cord that operate like a mixing board’s gain or volume control, enhancing or lessening sensations as they travel from the skin and other sensory organs to the brain. In certain ASD models, these second-order neurons seemed to be perpetually set to high, leading to sensory overload.
“It caused us to realize the potential to address sensory over-reactivity by decreasing the activity of sensory neurons, or the responsiveness of sensory neurons, in the peripheral nervous system,” he explained.
The most sensible approach would be employing medications that decrease sensory neuron activity. Lauren Orefice, a postdoctoral fellow in the Ginty lab at the time, believed that benzodiazepines could be used to inhibit nerve cells in the periphery to alleviate sensory over-reactivity. However, pediatricians are often hesitant to prescribe potentially addictive sedatives to young patients.
“Thus, one strategy we are pursuing is developing peripherally constrained benzodiazepines that can diminish the activity of neurons in the peripheral nervous system without impacting the brain, thus avoiding sedative side effects,” Ginty stated.
For children with hypersensitivity in autism, such a medication could be transformative. It has the potential to minimize overstimulation, reduce anxiety, prevent meltdowns, and allow them to perceive a hug as a joy rather than a source of discomfort.
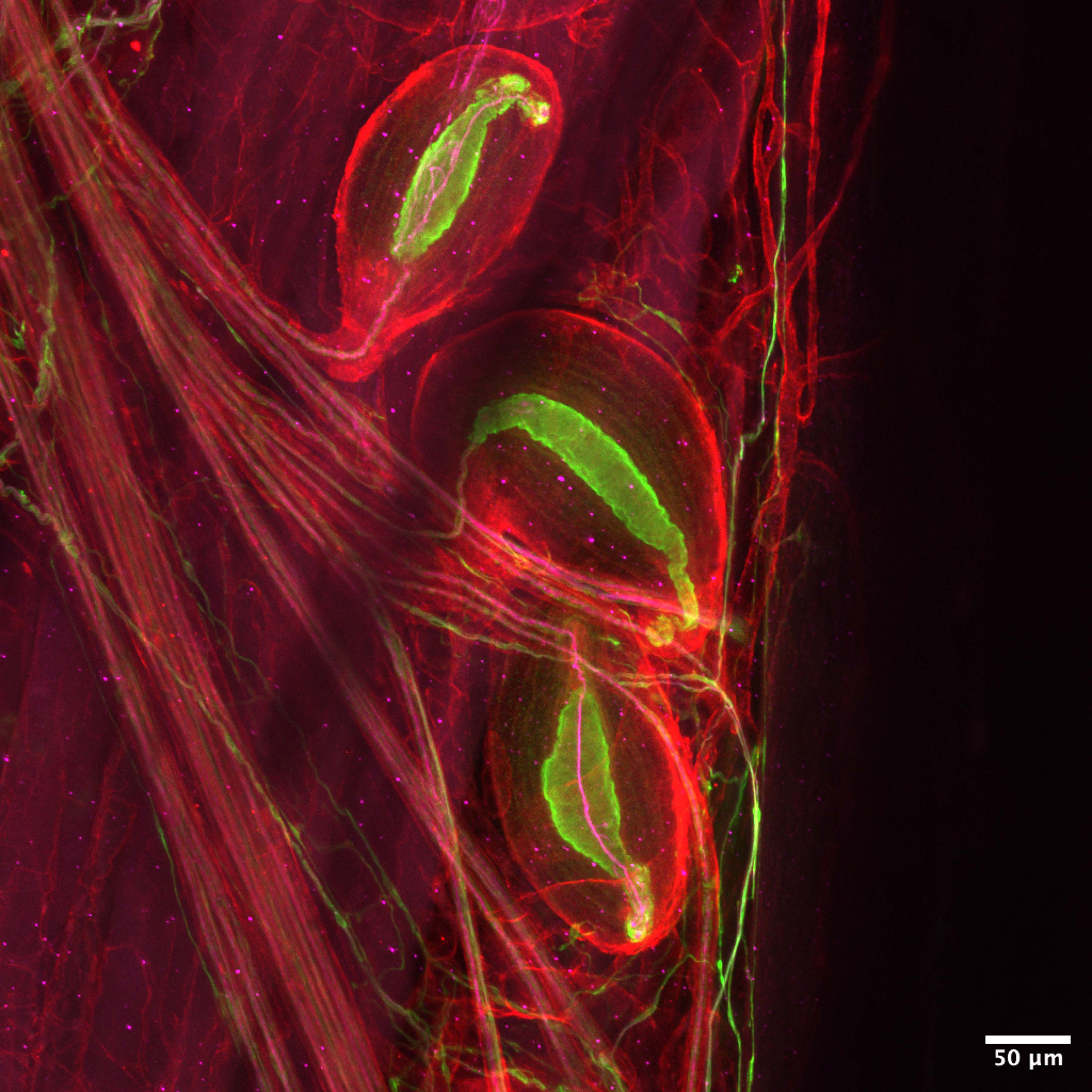
Pacinian corpuscles — neurons that detect vibrations — are sensitive enough to sense someone’s footsteps from across the room.
Image by Zoe Sarafis
The ramifications of Ginty’s research on the mechanisms surrounding pleasure and pain reach far beyond autism studies. The somatosensory system comprises around 20 different types of neurons dispersed throughout every conceivable area of the body: at the base of hair follicles, within the depths of our dermis, in our muscles and joints — anywhere that detects changes in stretch, pressure, vibration, temperature, and even our spatial orientation. If he had to choose a favorite neuron, he’d select two: Pacinian corpuscles and nociceptors.
Pacinian corpuscles detect vibrations. They are sufficiently sensitive to perceive footsteps from across the room, and powerful enough to evoke tears when music resonates within our bodies. Nociceptors respond to harmful stimuli — or, in simpler terms, pain.
“We’re uncovering how nociceptors connect within the central nervous system to produce reflexes, like swiftly withdrawing your hand from a hot surface, or to the emotional aspects of pain,” Ginty remarked. “These neurons are genuinely astonishing. They possess very high thresholds, unlike Pacinian corpuscles, which respond merely to slight movements or vibrations of the skin. Nociceptors only send an electrical signal when an injury occurs.”
Recent genetic tools enable Ginty to understand how nociceptors link with the central nervous system and identify every protein that nociceptors produce, unveiling a new spectrum of potential drug targets. “Currently, opioids are the most effective treatment we possess for various types of pain, and that’s indeed caused us significant issues,” he expressed. “We are intensively working to discover non-opioid strategies for managing pain and have identified numerous potential avenues by targeting the nociceptors.
“““html
themselves.
Ginty’s laboratory is not configured for pharmaceutical development. However, the investigations conducted in his lab provide the essential foundation that the drug industry requires to develop treatments that enhance lives. Ginty characterized his research as frequently exploratory. It is not consistently evident whether a specific experiment will evolve into a therapy or commercially viable drug, which explains the infrequent adequacy of industry funding. It is federal grants that have sustained the basic science which, ultimately, yields cures.
During the Trump administration’s feud with Harvard, Ginty had two grants frozen. The first, a collaboration with Clifford Woolf at Boston Children’s Hospital, was investigating how pain signals from the skin, joints, and bones are transmitted to the spinal cord and communicated to the brain, as well as pinpointing where in the brain those signals are directed.
The second was a notable R35 grant, often referred to as the Outstanding Investigator Award, which offers adaptable, long-term funding to veteran researchers enabling them to engage in particularly groundbreaking investigations. It was intended to finance the majority of Ginty’s work for eight years, but it was cut after just one year.
He stated that the most crippling aspect of the cancellations is their occurrence during a period of extraordinary advancement in neurobiology.
“The progress is simply amazing due to the synergy of integrating genetics, physiology, and molecular biology, with the knowledge being unveiled. Never before have advancements occurred at such a rapid pace and to such an extent as we’re witnessing now. I feel grateful to occupy this position and contribute to uncovering how the nervous system functions and exploring new therapeutic avenues. We must discover strategies to endure the current funding crisis to ensure that advancements leading to new treatments for nervous system disorders can persist.”
“““html

How a mere fishing expedition contributed to GLP-1
Narrative of transformative therapy highlights the essential role of primary research advancements

Combating Alzheimer’s through individual findings
‘I was merely pursuing the scientific path.’

Revising genetic fate
David Liu, recipient of the Breakthrough Prize, retraces his journey to a ‘remarkably thrilling’ disease combatant: ‘This embodies the core of basic science.’

Prolonged journey from 1992 revelation to 2024 Nobel
Gary Ruvkun shares years of exploration, which gradually attracted attention, primarily driven by NIH funding
“`