Zebrafish possess an extraordinary and uncommon capability to regenerate and mend their hearts following injury. Recent findings from Caltech and UC Berkeley have pinpointed the network of genes that governs this capability, providing insights into how a human heart might one day be restored after injury, including after a heart attack or in instances of congenital heart disorders.
This investigation was a partnership between the labs of Marianne Bronner, Caltech’s Edward B. Lewis Professor of Biology and head of the Beckman Institute, and developmental biologist Megan Martik from UC Berkeley. A manuscript detailing the research is published in the journal Proceedings of the National Academy of Sciences.
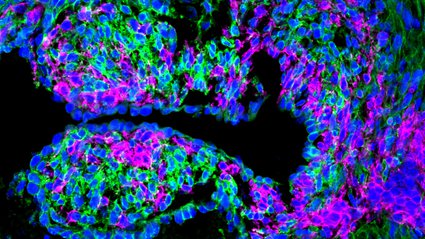
The heart consists of various types of cells, including muscle, nerve, and blood vessel cells. In zebrafish, approximately 12 to 15 percent of these cells arise from a unique group of stem cells known as neural crest cells. The Bronner and Martik labs have investigated neural crest cells and their vital function in the development of numerous laboratory animal models, such as zebrafish and lamprey. Similarly, humans have corresponding neural crest cells that generate diverse cell types in nearly every organ, including those in the facial structure and nervous system.
In this recent research, the team discovered that heart cells originating from neural crest cells play a critical role in managing the repair process in harmed zebrafish hearts. When those heart cells derived from neural crest cells were eliminated during experiments, the hearts lost their natural ability to regenerate after injury. Significantly, the study highlighted the intricate network of genes that are activated during the regeneration process. The researchers found these genes to be essential for standard embryonic development, subsequently inactivated during adulthood, but reactivated to facilitate tissue regeneration.
Moving forward, the team intends to investigate how these cells reactivate such gene programs to tackle the question: What signal prompts the activation of these genes following damage? Ultimately, this research could uncover whether humans could trigger similar genes if provided with that same signal. The Martik team is currently employing CRISPR technology—a prevalent gene-editing method—on human heart cells in laboratory cultures to assess if these genes can be reawakened.
The manuscript is titled “Reactivation of an Embryonic Cardiac Neural Crest Transcriptional Profile During Zebrafish Heart Regeneration.” The initial authors are Rekha M. Dhillon-Richardson and Alexandra K. Haugan from UC Berkeley. In addition to Bronner and Martik, other co-authors include Luke W. Lyons and Joseph K. McKenna from UC Berkeley. Financial support was provided by the American Heart Association, the Shurl and Kay Curci Foundation, the National Institutes of Health, and the National Science Foundation. Bronner is also an affiliated faculty member with the Tianqiao and Chrissy Chen Institute for Neuroscience at Caltech.