“`html
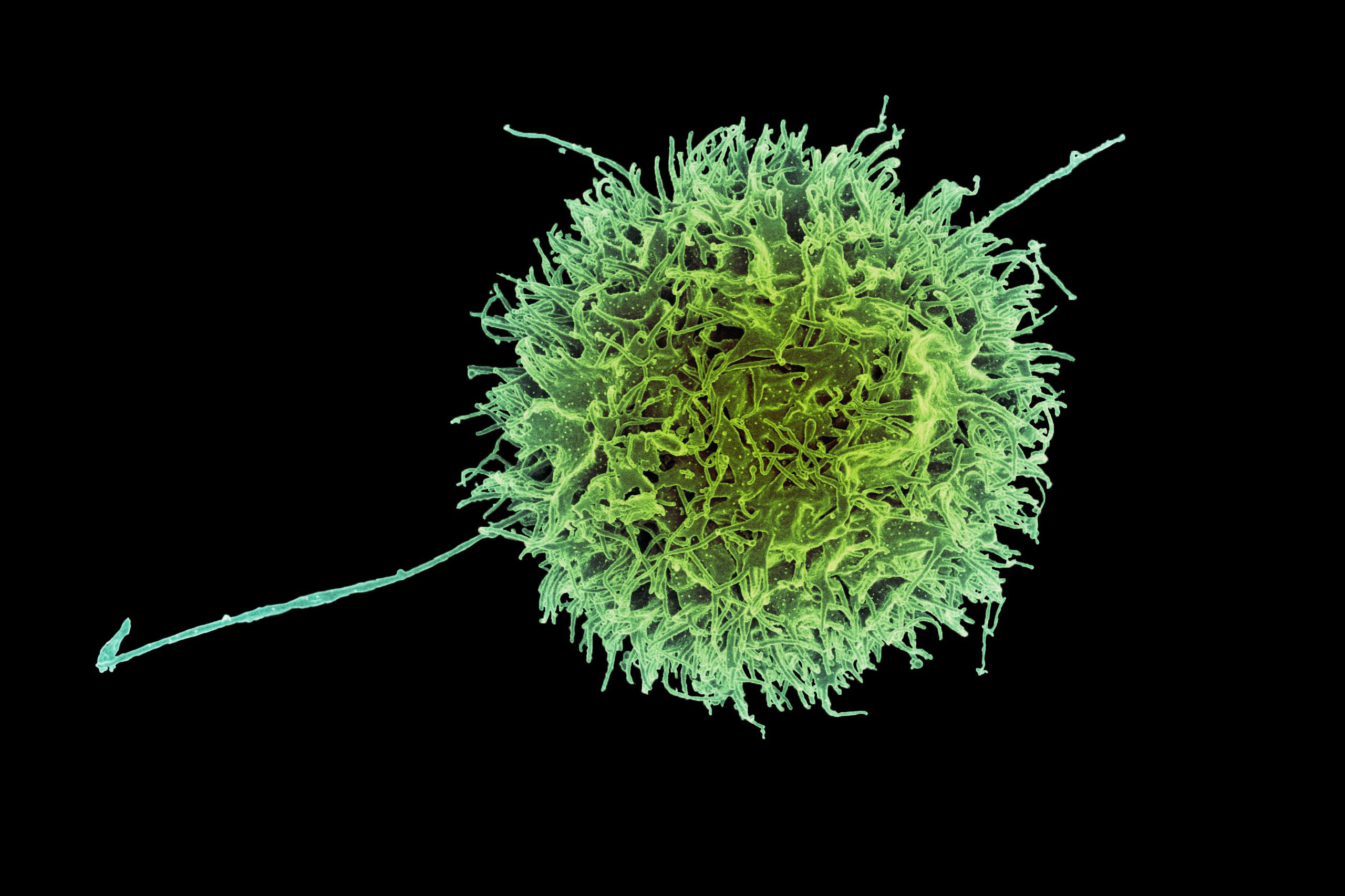
One of the latest armaments that researchers have designed against cancer is a form of modified immune cell referred to as CAR-NK (natural killer) cells. Analogous to CAR-T cells, these cells can be programmed to target malignancies.
Scientists from MIT and Harvard Medical School have now devised a novel method to modify CAR-NK cells, significantly reducing the likelihood of rejection by the patient’s immune system, which is a frequent limitation of this treatment type.
This new enhancement may also facilitate the development of “off-the-shelf” CAR-NK cells that could be administered to patients promptly upon diagnosis. Conventional methods for engineering CAR-NK or CAR-T cells typically require several weeks.
“This allows for one-step modification of CAR-NK cells that can escape rejection by host T cells and other immune cells. Furthermore, they effectively eliminate cancer cells and are safer,” states Jianzhu Chen, a biology professor at MIT, a member of the Koch Institute for Integrative Cancer Research, and one of the senior authors of the study.
In a study involving mice with humanized immune systems, the researchers demonstrated that these CAR-NK cells could eradicate most cancer cells while dodging the host immune system.
Rizwan Romee, an associate professor of medicine at Harvard Medical School and Dana-Farber Cancer Institute, is also a senior author on the paper, which is published today in Nature Communications. The lead author of the paper is Fuguo Liu, a postdoctoral researcher at the Koch Institute and a research fellow at Dana-Farber.
Evading the immune system
NK cells play a vital role in the body’s innate immune defenses, with their main duty being to locate and eliminate cancerous and virus-infected cells. One of their cytotoxic strategies, also utilized by T cells, is known as degranulation. Through this mechanism, immune cells release a protein termed perforin, which can create openings in another cell, leading to cell death.
To generate CAR-NK cells for treating cancer patients, clinicians first obtain a blood sample from the patient. NK cells are extracted from the sample and modified to express a protein known as a chimeric antigen receptor (CAR), which can be customized to target specific proteins present on cancer cells.
Subsequently, the cells undergo several weeks of proliferation until there are sufficient numbers to be reinfused into the patient. A comparable method is applied for creating CAR-T cells. Numerous CAR-T cell therapies have been authorized for treating blood cancers such as lymphoma and leukemia, but CAR-NK therapies remain in clinical trials.
Given the lengthy process required to cultivate a population of engineered cells suitable for infusion, and acknowledging that these cells may not be as viable as those derived from a healthy donor, researchers are investigating an alternative strategy: utilizing NK cells from a healthy individual.
Such cells could be cultivated in substantial amounts and would be available whenever required. Nevertheless, the drawback of using these cells lies in the possibility that the recipient’s immune system may recognize them as foreign and mount an attack before they can begin eliminating cancer cells.
In the recent study, the MIT team aimed to uncover a method to assist NK cells in “hiding” from a patient’s immune response. Through investigations of immune cell interactions, they found that NK cells could bypass a host T-cell reaction if they lacked surface proteins referred to as HLA class 1 proteins. Typically found on NK cell surfaces, these proteins can prompt T cells to attack if the immune system does not identify them as “self.”
To capitalize on this, the researchers modified the cells to express a sequence of siRNA (short interfering RNA) that disrupts the genes responsible for HLA class 1. They also introduced the CAR gene, alongside the gene for either PD-L1 or single-chain HLA-E (SCE). PD-L1 and SCE are proteins that enhance NK cell effectiveness by amplifying genes involved in the destruction of cancer cells.
All of these genes can be contained on a single DNA segment, known as a construct, simplifying the transformation of donor NK cells into immune-evasive CAR-NK cells. The researchers employed this construct to create CAR-NK cells aimed at a protein named CD-19, commonly found on cancerous B cells in lymphoma patients.
NK cells unleashed
The researchers assessed these CAR-NK cells in mice possessing a human-like immune system. These mice were also administered lymphoma cells.
Mice that received CAR-NK cells with the new construct sustained the NK cell population for a minimum of three weeks, and the NK cells were capable of almost completely eradicating the cancer in those mice. Conversely, in mice that received either NK cells without genetic modifications or NK cells with solely the CAR gene, the host immune cells attacked the donor NK cells. In these cases, the NK cells diminished within two weeks, allowing the cancer to proliferate unchecked.
The researchers also discovered that these engineered CAR-NK cells were significantly less likely to trigger cytokine release syndrome — a prevalent adverse effect of immunotherapy treatments, which can lead to life-threatening complications.
Due to the potentially enhanced safety profile of CAR-NK cells, Chen predicts that they could ultimately replace CAR-T cells. For any CAR-NK treatments currently in development targeting lymphoma or other cancer types, adapting them by incorporating the construct developed in this study should be feasible, he suggests.
The researchers now aspire to initiate a clinical trial of this method, collaborating with colleagues at Dana-Farber. They are also partnering with a local biotech firm to examine CAR-NK cells for the treatment of lupus, an autoimmune disease that causes the immune system to attack healthy tissues and organs.
The research was partially financed by Skyline Therapeutics, the Koch Institute Frontier Research Program through the Kathy and Curt Marble Cancer Research Fund and the Elisa Rah Memorial Fund, the Claudia Adams Barr Foundation, and the Koch Institute Support (core) Grant from the National Cancer Institute.
“`