“`html
Health
Can lithium clarify — and potentially treat — Alzheimer’s?
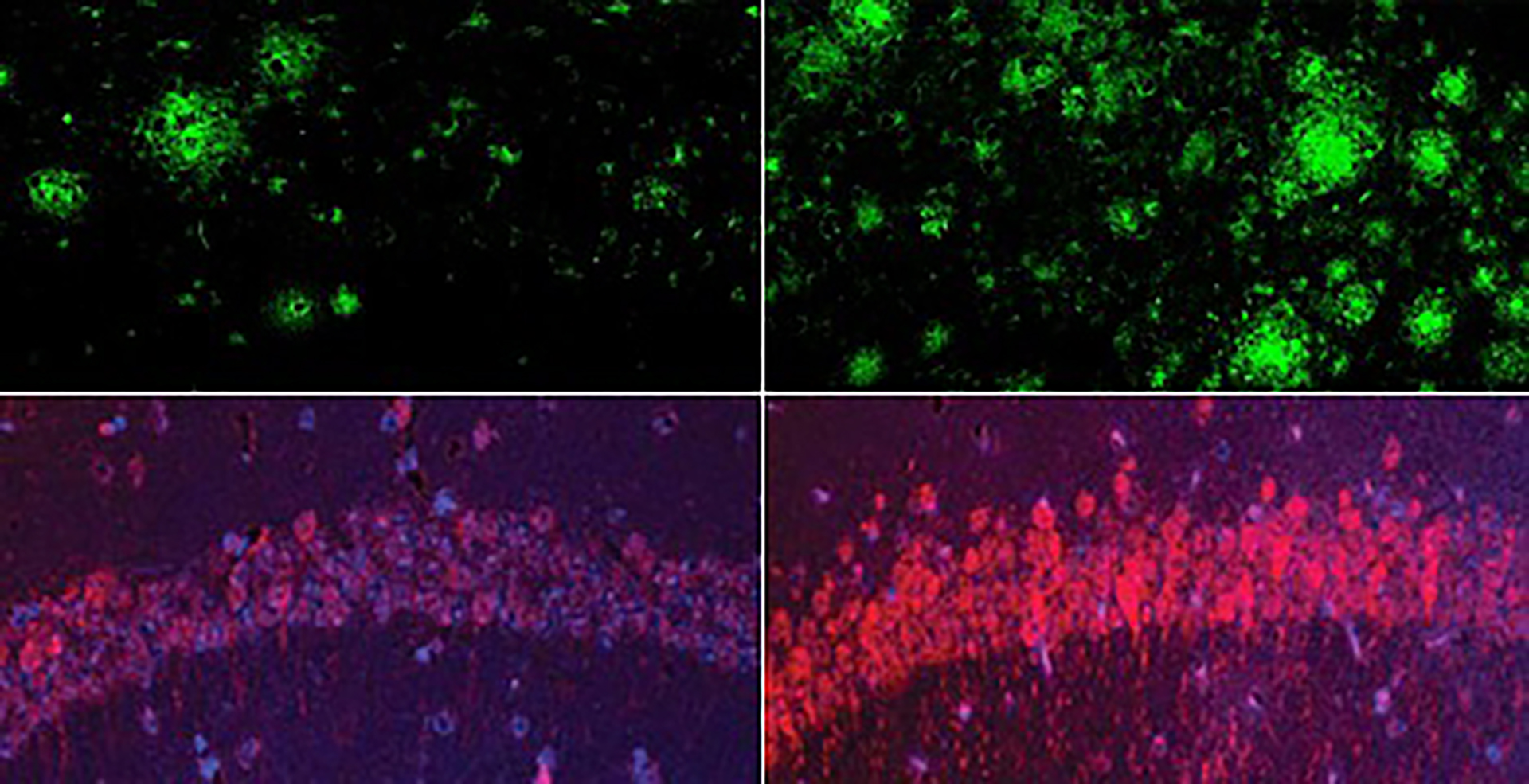
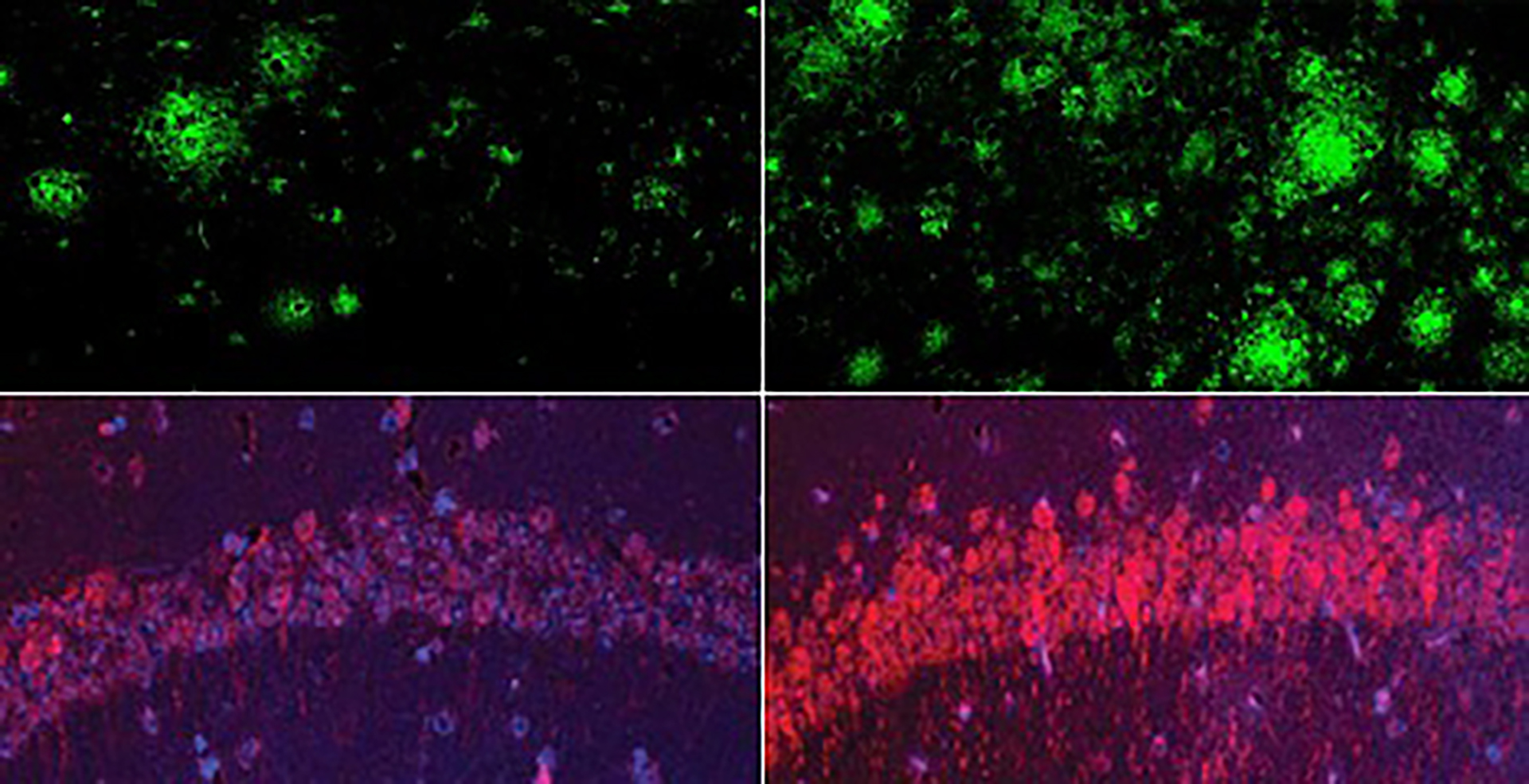
In a mouse model of Alzheimer’s disease, a shortage of lithium (right) greatly increased amyloid beta deposits in the brain when compared to mice with normal lithium levels (left). Bottom row: The same was true for the Alzheimer’s neurofibrillary tangle protein tau.
Yankner Lab
Research proposes a novel theory of the disease and a method for combating it
What is the initial spark that triggers the memory-eroding journey of Alzheimer’s disease? Why do certain individuals with Alzheimer’s-like alterations in their brains never progress to dementia? These inquiries have perplexed neuroscientists for years.
Recently, a group of investigators at Harvard Medical School may have uncovered a solution: lithium deficiency in the brain.
The publication, released Wednesday in Nature, reveals for the first time that lithium is naturally present in the brain, protecting it from neurodegeneration and preserving the typical function of all primary brain cell types. The discoveries — a decade in development — are founded on a sequence of experiments involving mice and evaluations of human brain tissue and blood samples from individuals at various stages of cognitive health.
The researchers determined that the loss of lithium in the human brain is one of the earliest transformations leading to Alzheimer’s, while in mice, similar depletion of lithium expedited brain pathology and memory deterioration. Furthermore, the team discovered that reduced lithium levels resulted from attachment to amyloid plaques and impaired absorption in the brain. In a concluding series of experiments, the team identified a unique lithium compound that bypasses absorption by amyloid plaques, which restored memory in mice.
The outcomes unify decades-long observations in patients, offering a fresh theory of the disease and a novel strategy for early diagnosis, prevention, and treatment.
Screening for lithium through standard blood tests may eventually provide a means to identify individuals at risk who could benefit from interventions to prevent or postpone Alzheimer’s onset.
Impacting an estimated 400 million individuals globally, Alzheimer’s disease encompasses a range of brain irregularities — such as accumulations of the protein amyloid-beta, neurofibrillary tangles of the protein tau, and decrease of a protective protein known as REST — yet these have never fully explained the entirety of the disease. For example, some individuals with such irregularities exhibit no signs of cognitive decline. Furthermore, recently developed therapies that focus on amyloid-beta generally do not reverse memory loss and only slightly decrease the rate of decline.
It is also evident that genetic and environmental factors influence the risk of Alzheimer’s, but researchers have yet to ascertain why some individuals with the same risk elements develop the disease while others do not.
Lithium, the study authors mentioned, might be a significant missing connection.
“The notion that lithium deficiency could be a precursor of Alzheimer’s disease is innovative and implies a different therapeutic pathway,” stated senior author Bruce Yankner, a professor of genetics and neurology in the Blavatnik Institute at HMS, who in the 1990s was the first to prove that amyloid-beta is harmful.
The study enhances optimism that researchers might one day employ lithium to treat the ailment in its entirety rather than concentrating on a singular aspect such as amyloid-beta or tau, he mentioned.
One of the principal findings in the research is that as amyloid-beta starts to create deposits in the early stages of dementia in both humans and mouse models, it binds with lithium, diminishing lithium’s efficacy in the brain. The decreased lithium levels influence all major types of brain cells and, in mice, lead to modifications mirroring Alzheimer’s disease, inclusive of memory impairment.
The authors highlighted a category of lithium compounds that can avoid being captured by amyloid-beta. Treating mice with the most potent amyloid-evading compound, known as lithium orotate, reversed Alzheimer’s disease pathology, averted brain-cell injury, and restored memory.
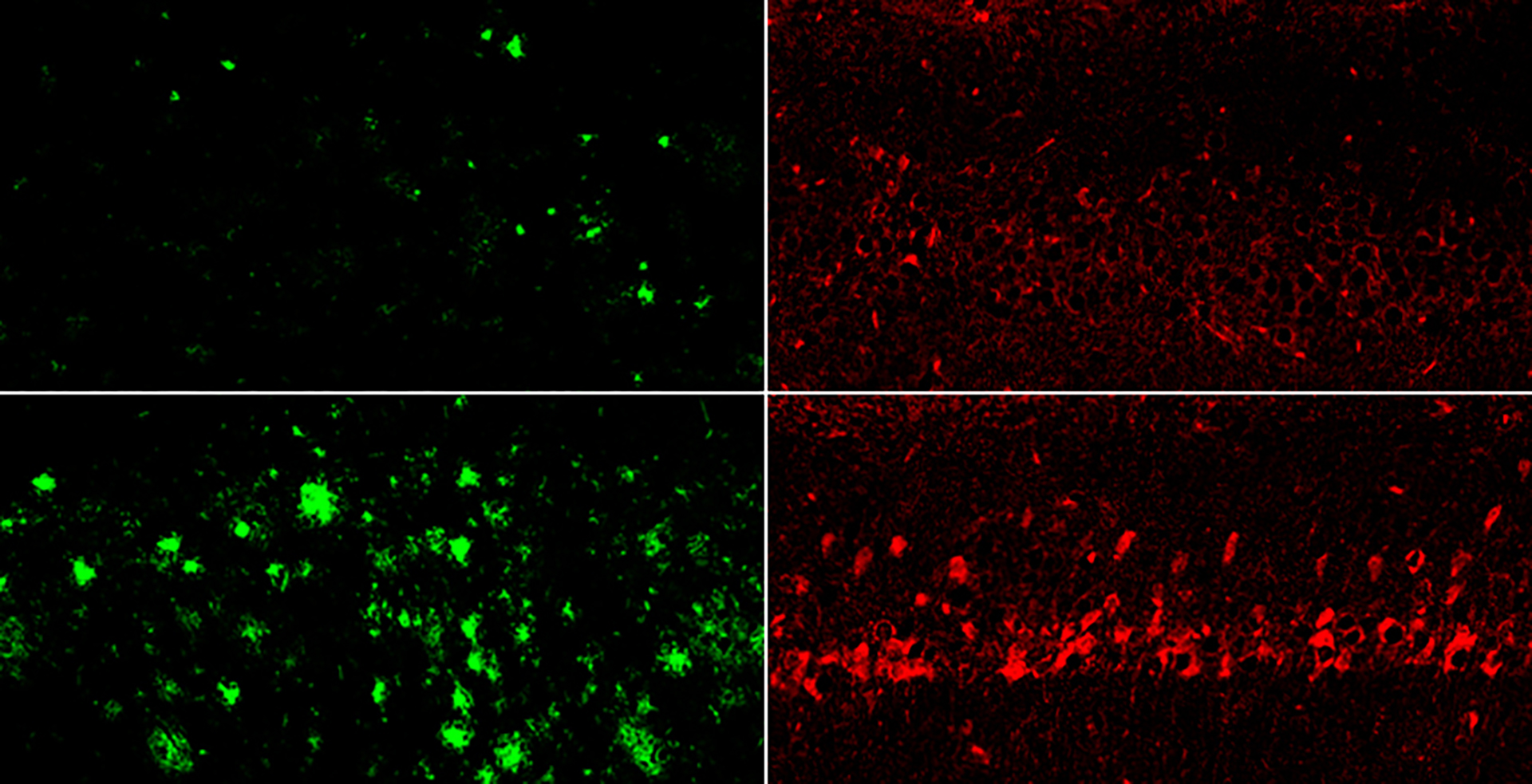
Administering mice with the amyloid-evading lithium orotate (top) significantly reduced both amyloid beta (left) and tau (right) more effectively than lithium carbonate (bottom).
Yankner Lab
While the discoveries require validation in humans through clinical trials, they indicate that monitoring lithium levels could assist in screening for early Alzheimer’s. Additionally, the findings underscore the significance of evaluating amyloid-evading lithium compounds for treatment or prevention.
Other lithium compounds are currently utilized to manage bipolar disorder and major depressive disorder; however, these are administered at much higher doses that may be toxic, particularly to older patients. Yankner’s team identified that lithium orotate is effective at one-thousandth of that dose — sufficient to replicate the natural lithium level in the brain. Mice treated for nearly their entire adult lives displayed no signs of toxicity.
“It is essential to be cautious about extrapolating from mouse models, and one cannot be certain until it is tested in a controlled human clinical trial,” Yankner noted. “But thus far, the outcomes are very promising.”
Lithium depletion is an early indicator of Alzheimer’s
Yankner developed an interest in lithium while utilizing it to investigate the neuroprotective protein REST. Uncovering whether lithium is present in the human
“`
the mind and if its concentrations alter as neurodegeneration unfolds and advances; nevertheless, this necessitated access to cerebral tissue, which typically cannot be obtained from living individuals.
Thus, the laboratory collaborated with the Rush Memory and Aging Project in Chicago, which possesses a collection of postmortem brain tissue contributed by thousands of study participants spanning the entire range of cognitive wellness and illness.
This variety was essential because attempting to examine the brain during the later phases of Alzheimer’s resembles surveying a battlefield post-conflict, according to Yankner; substantial destruction makes it difficult to discern the origins of the issue. However, in the initial stages, “prior to significant brain damage, one can gather critical insights,” he stated.
Under the leadership of first author Liviu Aron, a senior research associate at the Yankner Lab, the team utilized an advanced form of mass spectroscopy to assess minute levels of approximately 30 different metals in the brains and blood of cognitively healthy individuals, those in the early phase of dementia known as mild cognitive impairment, and individuals with severe Alzheimer’s.
Lithium was the solitary metal that exhibited significantly varied levels across groups and altered during the earliest stages of memory decline. Its concentrations were elevated in the cognitively healthy donors but substantially reduced in those facing mild impairment or full-blown Alzheimer’s.
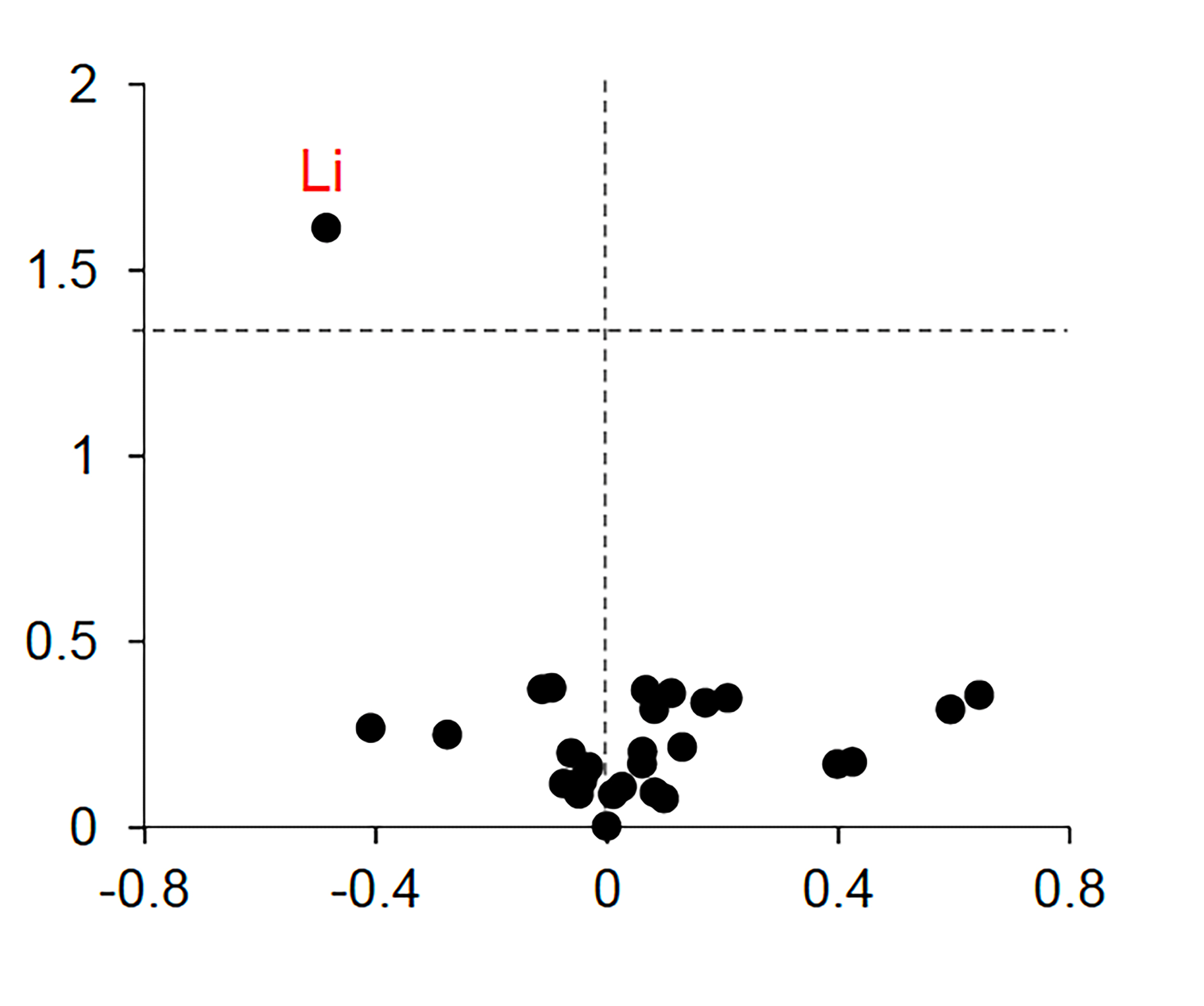
The team repeated the observations in samples obtained from numerous brain banks across the country.
This finding corresponded with prior population studies indicating that elevated lithium levels in the environment, including drinking water, correlated with reduced rates of dementia.
However, the new research extended beyond by directly observing lithium within the brains of individuals not treated with lithium, establishing a range that denotes normal levels, and illustrating that lithium plays a crucial role in brain health.
“Lithium is akin to other nutrients sourced from our surroundings, such as iron and vitamin C,” Yankner remarked. “This is the first occasion anyone has demonstrated that lithium exists at a natural, biologically significant level without being administered as a medication.”
Then Yankner and his team advanced their research. They proved in mice that lithium deficiency is not simply associated with Alzheimer’s disease—it actively contributes to its progression.
Loss of lithium triggers a spectrum of Alzheimer’s-related alterations
The researchers discovered that providing healthy mice with a lithium-restricted diet lowered their brain lithium levels to those comparable to patients with Alzheimer’s. This appeared to expedite the aging process, resulting in brain inflammation, deterioration of synaptic connections among neurons, and cognitive decline.
In Alzheimer’s mouse models, diminished lithium significantly hastened the formation of amyloid-beta plaques and structures resembling neurofibrillary tangles. Lithium deficiency also activated inflammatory brain cells known as microglia, impairing their ability to break down amyloid; caused loss of synapses, axons, and the protective myelin surrounding neurons; and accelerated cognitive deterioration and memory loss—all defining features of Alzheimer’s disease.
The experiments on mice further revealed that lithium influenced the activity of genes recognized for increasing or decreasing the risk of Alzheimer’s, including the most notable, APOE.
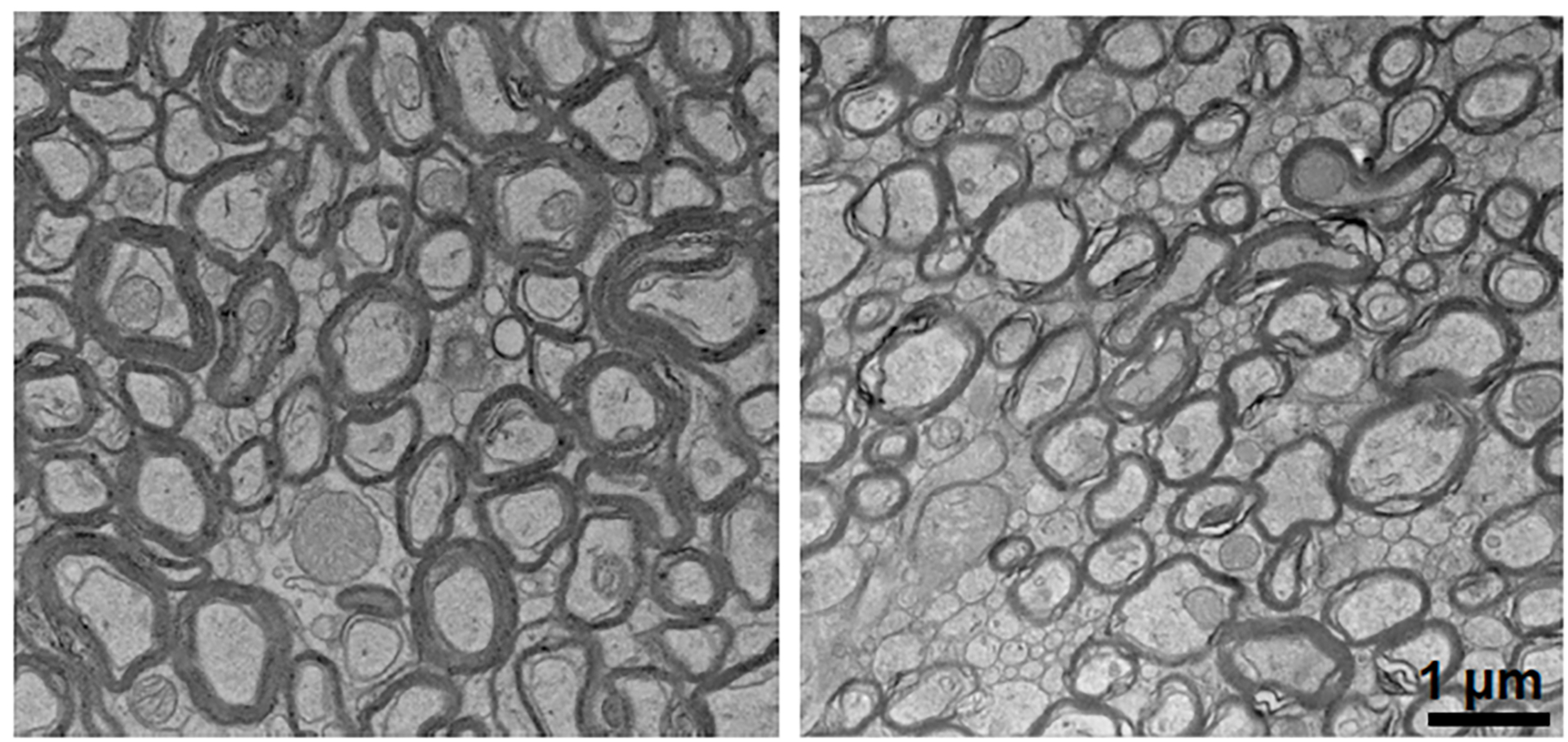
Lithium deficiency resulted in reduced myelin surrounding neurons (right) when compared to normal mice (left).
Yankner Lab
Replenishing lithium through lithium orotate in the mice’s drinking water reversed the disease-related damage and restored memory function, even in aged mice with advanced conditions. Importantly, sustaining consistent lithium levels in early life thwarted the onset of Alzheimer’s—a revelation affirming that lithium drives the disease process.
“What astonishes me most about lithium is its extensive impact on the multiple manifestations of Alzheimer’s. Throughout my years of research on this disease, I have not encountered anything quite like it,” stated Yankner.
A promising direction for Alzheimer’s therapy
A few limited clinical trials of lithium for Alzheimer’s disease have demonstrated some effectiveness; however, the lithium formulations employed—like the clinical standard, lithium carbonate—may be harmful to older adults at the high doses typically used in clinics.
The recent research clarifies why: Amyloid-beta was trapping these other lithium formulations before they could exert their effects. Yankner and his colleagues identified lithium orotate by developing a screening platform that scans a compound library for those potentially able to bypass amyloid-beta. Other researchers can now utilize this platform to discover additional lithium compounds that evade amyloid and may be even more potent.
“One of the most inspiring discoveries for us was that there were significant impacts even at this remarkably low dosage,” said Yankner.
If verified in subsequent studies, the researchers propose that lithium screening through routine blood tests may someday provide a method to identify vulnerable individuals who could benefit from treatment to avert or delay the onset of Alzheimer’s.
Examining lithium levels in individuals who resist Alzheimer’s as they age may assist scientists in determining a target level that could be maintained to prevent the disease’s emergence, remarked Yankner.
Since lithium has not yet been proven safe or effective in safeguarding against neurodegeneration in humans, Yankner stresses that individuals should refrain from independently consuming lithium compounds. However, he voiced cautious optimism that lithium orotate or a similar compound will advance into clinical trials soon and could eventually transform the narrative of Alzheimer’s treatment.
“My aspiration is that lithium will enact something more fundamental than anti-amyloid or anti-tau therapies, not merely mitigating but reversing cognitive deterioration and enhancing patients’ quality of life,” he expressed.
This inquiry was supported by the National Institutes of Health.