Management of persistent pain continues to depend significantly on opioids. Although they are effective, these substances are extremely habit-forming and can be fatal if abused. In an effort to create a safe and effective substitute for opioids, investigators at Washington University School of Medicine in St. Louis and Stanford University have formulated a substance that imitates a naturally occurring molecule found in the cannabis plant, leveraging its pain-relieving characteristics without inducing dependency or cognitive-altering side effects in mice.
Though further research is necessary, the substance displays potential as a non-addictive analgesic that could assist the estimated 50 million individuals in the U.S. experiencing chronic pain. The findings were published on March 5 in Nature.
“There is a pressing necessity to discover non-addictive solutions for chronic pain, and that has been a primary objective of my laboratory for the last 15 years,” stated the study’s senior author Susruta Majumdar, a professor of anesthesiology at WashU Medicine. “The specially designed compound we developed binds to pain-relieving receptors in the body but, by design, it cannot access the brain. This characteristic means the substance avoids psychoactive side effects like mood alterations and is not addictive as it does not interact with the brain’s reward pathway.”
Opioids alleviate pain sensations in the brain and take control of the brain’s reward mechanism, resulting in dopamine release and feelings of gratification, which contribute to their addictive nature. In spite of widespread public health alerts and media coverage of the risks associated with opioid addiction, countless overdose fatalities still take place. In 2022, approximately 82,000 deaths in the U.S. were attributed to opioids. This situation has prompted scientists to earnestly seek alternative pain management strategies.
“For centuries, individuals have sought relief from pain through marijuana,” explained co-corresponding author Robert W. Gereau, the Dr. Seymour and Rose T. Brown Professor of Anesthesiology and director of the WashU Medicine Pain Center. “Clinical trials have also assessed whether cannabis offers sustained pain relief. However, the psychoactive effects of cannabis have been a complication, hindering its acceptance as a viable pain treatment. Nevertheless, we managed to resolve that dilemma.”
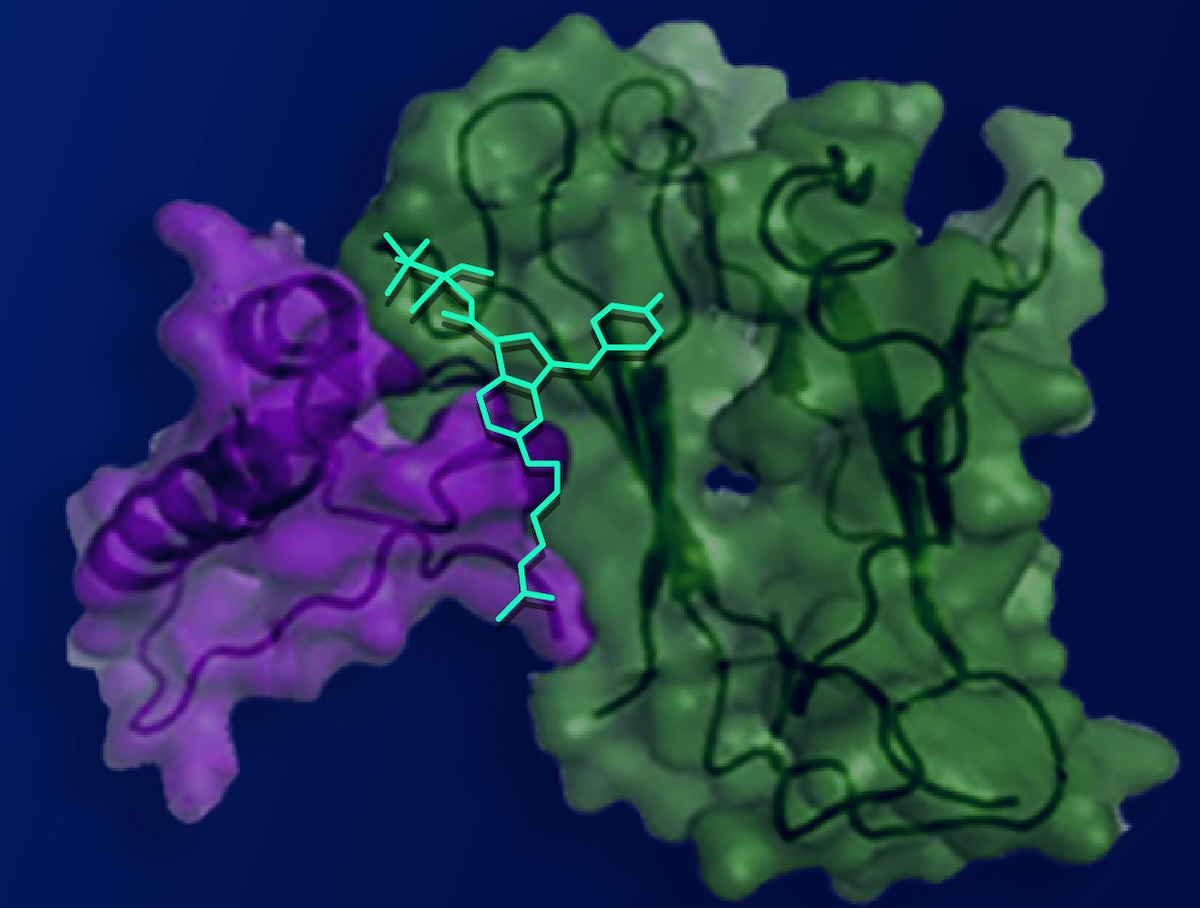
The psychoactive effects of marijuana are derived from natural compounds known as cannabinoid molecules found in the cannabis plant. These compounds bind to a receptor known as cannabinoid receptor one (CB1), located on the surface of brain cells and on pain-detecting nerve cells throughout the body.
In collaboration with researchers from Stanford University, co-first author Vipin Rangari, a WashU Medicine postdoctoral research associate in Majumdar’s lab, engineered a cannabinoid molecule with a positive charge, which prevents it from crossing the blood-brain barrier while enabling it to interact with CB1 receptors in other parts of the body. By altering the molecule to exclusively attach to pain-sensing nerve cells outside the brain, the researchers provided pain alleviation without cognitive-altering side effects.
The team tested the modified synthetic cannabinoid compound in mouse models simulating nerve injury pain and migraine headaches, assessing hypersensitivity to touch as an indicator of pain. Subjecting the mice to typically non-painful stimuli enables researchers to indirectly evaluate pain responses. In both models, injections of the modified compound eradicated touch hypersensitivity.
For numerous analgesics, particularly opioids, developing tolerance over time can impede their long-term effectiveness, necessitating increased dosages to reach similar pain relief levels. In this research, the adjusted compound provided extended pain relief — the animals exhibited no signs of developing tolerance despite receiving the compound twice daily over nine days. This is an encouraging indication that the molecule could function as a non-addictive remedy for chronic pain, which requires ongoing management.
The absence of tolerance to the compound stemmed from its tailored design. Collaborators at Stanford conducted advanced computational modeling that uncovered a concealed pocket on the CB1 receptor that could serve as an alternative binding site. This hidden pocket, validated by structural analysis, leads to decreased cellular activity related to tolerance development in contrast to the conventional binding site, although it was thought to be unreachable for cannabinoids. The researchers discovered that the pocket is accessible for brief intervals, allowing the modified cannabinoid compound to bind, thereby minimizing tolerance.
Creating molecules that alleviate pain with minimal side effects poses significant challenges, according to Majumdar. The researchers aim to further refine the compound into an oral medication that could undergo evaluation in clinical trials.
Rangari VA, O’Brien ES, Powers AS, Slivicki RA, Bertels Z, Appourchaux K, Aydin D, Ramos-Gonzalez N, Mwirigi I J, Lin L, Mangutov E, Sobecks BL, Awad-Agbaria Y, Uphade MB, Aguilar J, Peddada TN, Shiimura Y, Huang XP, Folarin-Hines J, Payne M, Kalathil A, Varga BR, Kobilka BK, Pradhan AA, Cameron MD, Kumar KK, Dror RO, Gereau IV RW, and Majumdar S. A cryptic pocket in CB1 drives peripheral and functional selectivity. Nature. March 5, 2025. DOI: 10.1038/s41586-025-08618-7
This research was funded by the National Institutes of Health (NIH), grant numbers R34NS126036, F32DA051160 and K99DA056691; the PhRMA Foundation Postdoctoral Fellowship, grant number 100001797. The content reflects solely the authors’ views and does not necessarily represent the official perspectives of the NIH.
About Washington University School of Medicine
WashU Medicine serves as a global frontrunner in academic medicine, encompassing biomedical research, patient care, and educational initiatives, with a faculty of 2,900. Its portfolio of research funding from the National Institutes of Health (NIH) ranks second largest among U.S. medical schools and has increased by 56% in the past seven years. In combination with institutional investments, WashU Medicine allocates over $1 billion annually to basic and clinical research innovation and training. The faculty practice consistently ranks among the top five in the nation, comprising more than 1,900 faculty physicians working at 130 sites, who also serve as the medical staff for Barnes-Jewish and St. Louis Children’s hospitals of BJC HealthCare. WashU Medicine boasts a rich legacy in MD/PhD training, having recently committed $100 million to scholarship opportunities and curriculum enhancements for medical students, and hosts exceptional training programs across all medical subspecialties as well as in physical therapy, occupational therapy, and communication sciences and audiology.
Originally published on the WashU Medicine website
The post Compound harnesses cannabis’ pain-relieving properties without side effects appeared first on The Source.