Every person who has ever existed began as a single cell, which then split innumerable times to form a body composed of approximately 10 trillion cells. These cells lead active lives, performing a variety of lively actions: contracting whenever we use a muscle, moving toward an injury site, and rhythmically pulsing for decades.
Cells exemplify active matter. While inanimate objects must consume fuel to move, such as airplanes and automobiles, active matter is similarly energized by its utilization of energy. The fundamental molecule for cellular energy is adenosine triphosphate (ATP), which facilitates chemical reactions allowing cellular mechanisms to function.
Researchers at Caltech have now created a bioengineered coordinate framework to observe the movement of cellular mechanisms. This research enhances the understanding of how cells organize chaos, particularly during embryonic development and the systematic movements of chromosomes essential for accurate cell division.
The study was carried out in the labs of Rob Phillips, the Fred and Nancy Morris Professor of Biophysics, Biology, and Physics, and Matt Thomson, Professor of Computational Biology and Investigator at the Heritage Medical Research Institute. A paper detailing this research appears in the journal Proceedings of the National Academy of Sciences.
The core components of cellular machinery are motors and filaments constructed from proteins, which serve as the cell’s muscles and skeleton. These structures autonomously assemble, akin to tiny protein robots, facilitating cell movement. In 2018, former graduate student Tyler Ross (PhD ’21) devised a system of these elements that can be manipulated with light in a laboratory environment, allowing scientists to monitor and experiment with their movements. Each experimental apparatus measures only the width of a human hair, housing thousands of individual motors and filaments.
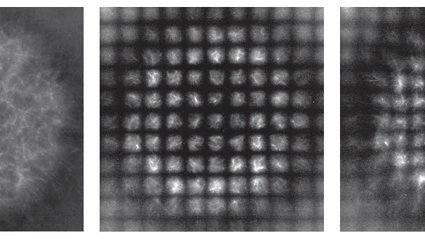
In this new endeavor, directed by former graduate student Soichi Hirokawa (PhD ’23), the team formulated additional light patterns that establish a grid, or coordinate system, across the mixture of motors and filaments. To visualize this, think of a sheet of rubber with a grid design on it—when the rubber is stretched and deformed, so too is the grid. Initially a set of evenly spaced squares, the grid’s distortion indicates which areas are being extended or compressed, and to what extent. Thus, the team is able to track the movements of many filaments and motors—they are too minute to be observed directly, but the light-patterned grid, each square measuring approximately 12-by-12 micrometers, is visible under a microscope.
“The system permits us to observe how these biomolecules restructure as they collectively assemble a structure,” notes Hirokawa. “With it, we can differentiate the processes that contribute to the deformities visible on these squares.”
This innovative system enabled the team to quantify the competing dynamics of active shrinking versus a process that affects cellular self-assembly, known as diffusion. By mixing motors and filaments, the researchers initiated the components to contract inward, resembling a diminishing circle. Yet, each element inevitably undergoes some random movement or diffusion, jittering in various directions as the entire setup contracts. The deforming coordinate framework allowed the team to observe this interplay between active contraction and random diffusion, characterizing it thoroughly. Notably, they discovered that an increase in ATP within the system leads to greater random diffusion of the molecules.
“The emergence of patterns and structures in biology must contend with this randomness,” states Phillips. “The system is capable of organizing itself despite the chaotic forces at play.”
The dynamic coordinate system introduced here could find applications in various other contexts as well.
“Order is especially critical in processes like embryonic development,” explains staff scientist and co-author Heun Jin Lee. “An early embryo undergoes gastrulation, folding into a tube that ultimately becomes the digestive tract. One could envision decorating the surface of an embryo with a coordinate system that adapts as the embryo folds.”
The paper is titled “Motor-driven microtubule diffusion in a photobleached dynamical coordinate system.” In addition to Hirokawa, Lee, Thomson, and Phillips, Caltech co-authors include former graduate student Rachel Banks (PhD ’22), graduate student Ana Duarte, and postdoctoral scholar Bibi Najma. Funding was made possible by the Maximizing Investigators Research Awards and the Foundational Questions Institute. Matt Thomson is also an affiliated faculty member with the Tianqiao and Chrissy Chen Institute for Neuroscience at Caltech.