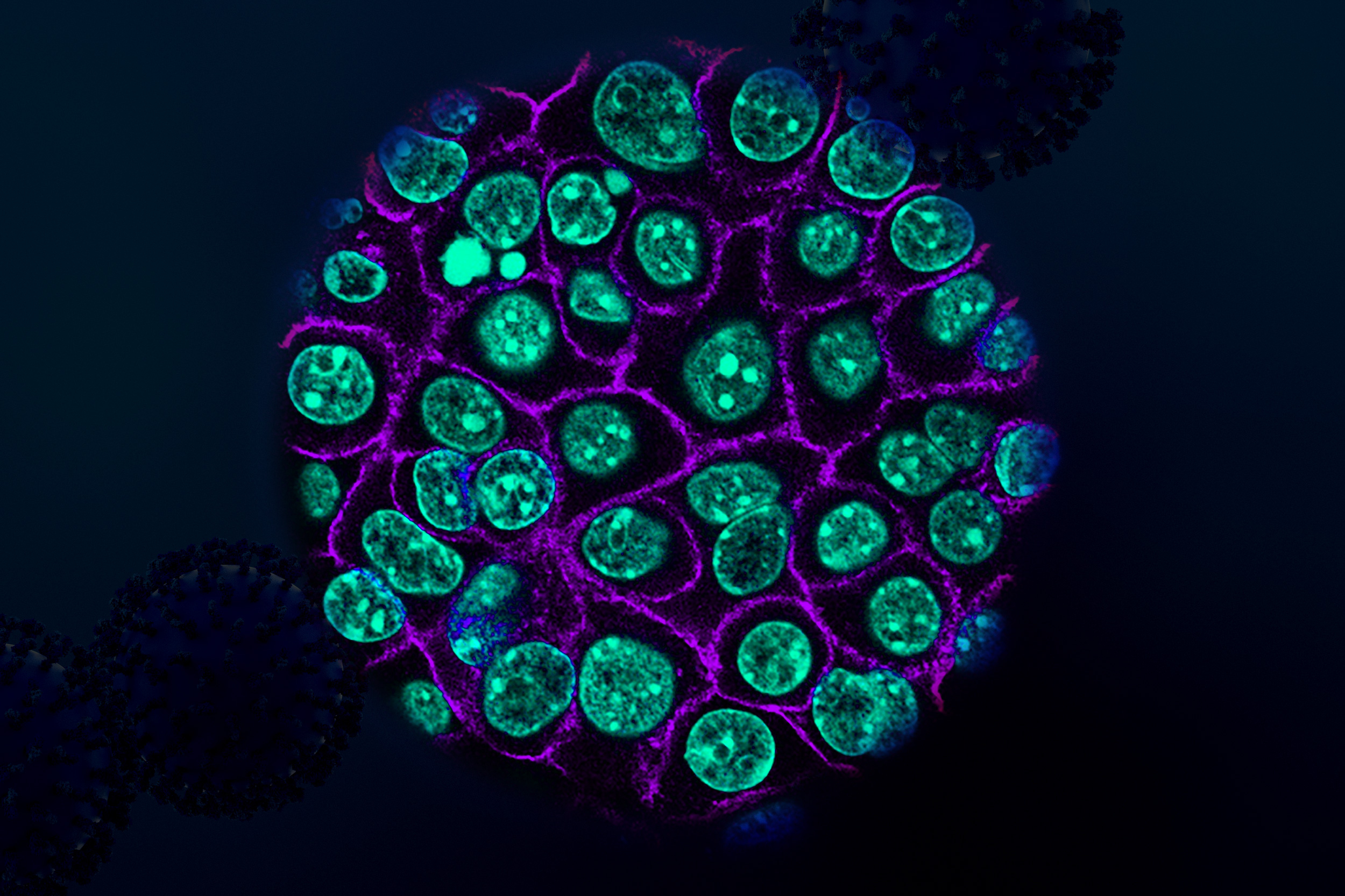
Scientists from MIT and Dana-Farber Cancer Institute have unveiled that a group of peptides expressed in pancreatic cancer cells may serve as a promising target for T-cell therapies and alternate strategies that combat pancreatic tumors.
Termed cryptic peptides, these molecules arise from sequences in the genome previously believed to be non-coding for proteins. While such peptides can also be detected in some healthy cells, this research identified approximately 500 that seem exclusive to pancreatic tumors.
Moreover, the researchers demonstrated the ability to create T cells that specifically target these peptides. These T cells successfully attacked pancreatic tumor organoids derived from patient cells, significantly hindering tumor growth in a mouse study.
“Pancreatic cancer remains among the most difficult cancers to treat. This research highlights an unforeseen vulnerability within pancreatic cancer cells that we may leverage therapeutically,” states Tyler Jacks, the David H. Koch Professor of Biology at MIT and a member of the Koch Institute for Integrative Cancer Research.
Jacks, alongside William Freed-Pastor, a physician-scientist at the Hale Family Center for Pancreatic Cancer Research at Dana-Farber Cancer Institute and an assistant professor at Harvard Medical School, are the senior authors of the study published today in Science. Lead authors of the paper include Zackery Ely PhD ’22 and Zachary Kulstad, a former research technician at Dana-Farber Cancer Institute and the Koch Institute.
Cryptic peptides
Pancreatic cancer possesses one of the lowest survival rates among cancers—only around 10 percent of patients survive five years post-diagnosis.
The majority of pancreatic cancer patients undergo a blend of surgery, radiation therapy, and chemotherapy. Immunotherapy strategies like checkpoint blockade inhibitors, intended to stimulate the body’s own T cells against tumors, are frequently ineffective on pancreatic tumors. Conversely, therapies utilizing engineered T cells to target tumors have shown promise in clinical trials.
These therapies involve programming the T-cell receptor (TCR) of T cells to recognize specific peptides or antigens present on tumor cells. Various ongoing efforts aim to pinpoint the most effective targets, and some promising antigens have been identified, consisting of mutated proteins frequently observed in sequenced pancreatic cancer genomes.
In the recent study, the MIT and Dana-Farber team sought to expand this investigation into tissue samples from pancreatic cancer patients, employing immunopeptidomics—a method that extracts peptides displayed on cell surfaces, followed by their identification through mass spectrometry.
Using tumor samples from about a dozen patients, the researchers crafted organoids—three-dimensional structures that partially replicate pancreatic architecture. The immunopeptidomics analysis, guided by Jennifer Abelin and Steven Carr at the Broad Institute, revealed that most novel antigens discovered within the tumor organoids were cryptic antigens. While cryptic peptides have been noted in other tumor types, this marks the inaugural discovery in pancreatic tumors.
Each tumor displayed an average of around 250 cryptic peptides, resulting in the identification of nearly 1,700 cryptic peptides overall.
“As we began receiving the data, it became evident that this was by far the most prevalent novel class of antigens, prompting our focus on this area,” Ely remarks.
The researchers subsequently analyzed healthy tissues to determine if any of these cryptic peptides appeared in normal cells. They found that nearly two-thirds could also be detected in at least one type of healthy tissue, leaving about 500 that seemed exclusive to pancreatic cancer cells.
“These are the candidates we believe could serve as excellent targets for future immunotherapies,” Freed-Pastor states.
Programmed T cells
To evaluate the viability of these antigens as targets for T-cell-based therapies, the researchers exposed approximately 30 cancer-specific antigens to immature T cells, discovering that 12 of them could induce significant populations of T cells aimed at those antigens.
The researchers then engineered a new batch of T cells to express those T-cell receptors. These modified T cells were able to annihilate organoids derived from patient pancreatic tumor cells. Moreover, when the researchers implanted the organoids into mice and subsequently treated them with the engineered T cells, tumor growth was markedly reduced.
This represents the first instance of T cells targeting cryptic peptides being used to eliminate pancreatic tumor cells. While total eradication of tumors was not achieved, the findings are promising, and it is likely that the T cells’ lethality could be enhanced in future research, according to the team.
Freed-Pastor’s lab is also initiating work on a vaccine aimed at certain cryptic antigens, which could assist in activating patients’ T cells to combat tumors expressing those antigens. Such a vaccine may incorporate a selection of the antigens pinpointed in this study, including those frequently observed across multiple patients.
This study may also assist researchers in developing additional therapy types, such as T cell engagers—antibodies designed to bind an antigen on one side and T cells on the other, facilitating the redirection of any T cell to eliminate tumor cells.
Any prospective vaccine or T cell therapy is likely a few years away from patient testing, as indicated by the researchers.
The research received funding in part from the Hale Family Center for Pancreatic Cancer Research, the Lustgarten Foundation, Stand Up To Cancer, the Pancreatic Cancer Action Network, the Burroughs Wellcome Fund, a Conquer Cancer Young Investigator Award, the National Institutes of Health, and the National Cancer Institute.