“`html
A novel technology that utilizes artificial intelligence (AI) to evaluate mammograms and enhance the precision of forecasting a woman’s individual five-year risk of developing breast cancer has obtained Breakthrough Device designation from the Food and Drug Administration (FDA). Created by scholars at Washington University School of Medicine in St. Louis, this software has been licensed to Prognosia Inc., a startup affiliated with WashU.
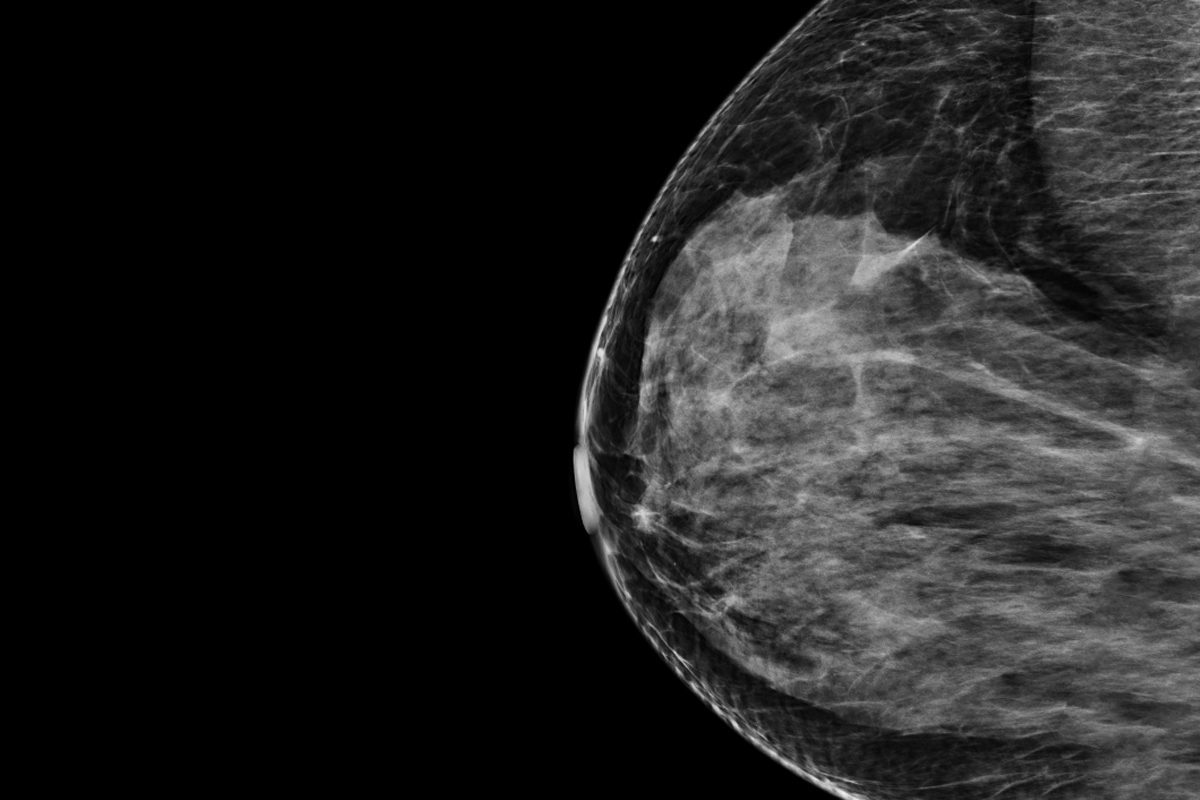
The system evaluates mammograms to generate a risk score that estimates the probability of a woman developing breast cancer within the next five years. The technology is compatible with both types of available mammogram imaging: the four 2D views provided by full-field digital mammography and the synthetic 3D representation generated by digital breast tomosynthesis. Significantly, the system yields an absolute five-year risk, allowing for a comparison between a woman’s risk and the national average based on breast cancer incidence rates. This offers a relevant estimate aligned with U.S. national risk reduction guidelines, enabling clinicians to make informed decisions if a woman’s risk is heightened.
The FDA’s Breakthrough Device designation accelerates the review process for complete market approval, aiming to provide patients and clinicians with quicker access to innovative medical devices. Products granted this designation have previously undergone extensive testing and demonstrated considerable potential to enhance treatment or diagnosis of serious, life-threatening conditions.
The software suite, named Prognosia Breast, was developed by Graham A. Colditz, MD, DrPH, the Niess-Gain Professor of Surgery at WashU Medicine and associate director of prevention and control at Siteman Cancer Center, located at Barnes-Jewish Hospital and WashU Medicine; and Shu (Joy) Jiang, PhD, an associate professor of surgery within the Division of Public Health Sciences in the Department of Surgery at WashU Medicine. Colditz and Jiang co-established Prognosia in 2024 in collaboration with WashU’s Office of Technology Management (OTM) and BioGenerator Ventures, the latter providing both financial backing and business strategy guidance from Entrepreneur-in-Residence David Smoller, PhD.

The software is a pre-trained machine learning system that examines mammogram images and provides an estimation of how likely a patient is to develop breast cancer within the coming five years, relying solely on images and the woman’s age. According to the developers, Prognosia Breast forecasts a person’s five-year risk of developing breast cancer 2.2 times more accurately than the traditional method, which is based on questionnaires that consider factors such as age, ethnicity, and family history. The system was trained using past mammograms from tens of thousands of patients who underwent breast cancer screenings at the Siteman Cancer Center. Some of them later developed cancer, helping the system learn what to identify in the earliest stages of tumor growth. Early indicators of illness may not even be visible to an experienced human eye.
“We’re thrilled about the potential of this technology to advance risk prediction and breast cancer prevention widely, regardless of where a woman is screened,” Colditz remarked. “The overarching goal is to make this technology accessible to any woman undergoing a screening mammogram anywhere globally. Regardless of the imaging method they receive, our data indicates the software’s capability to discern women at elevated risk of developing breast cancer over the next five years, empowering them to take targeted measures to mitigate that risk.”
This new device could significantly influence risk prediction since the necessary infrastructure is already established to start utilizing the software wherever mammography is offered. Moreover, many women routinely undergo mammograms. According to 2023 survey data from the Centers for Disease Control and Prevention, over 75% of women aged 50 to 74 reported having a mammogram in the past two years.
Even with extensive screening, approximately 34% of breast cancer patients in the U.S. are diagnosed at more advanced stages of the disease. The researchers believe that the ability to evaluate risk up to five years prior to cancer onset is likely to enhance early detection, thereby decreasing the incidence of late-stage cancer diagnoses. Early identification has proven to increase the effectiveness of treatment and lower mortality rates from breast cancer.
“Achieving Breakthrough Device designation serves as a significant affirmation of the remarkable commitment and vision of this research team to enhance breast cancer diagnosis and treatment,” stated Doug E. Frantz, PhD, vice chancellor for innovation and commercialization at WashU. “It takes years of focused effort to develop software that could seamlessly integrate into the workflow of any mammography facility, significantly enhancing the clinical value of routine mammograms, wherever they are conducted. This exemplifies the pivotal role of entrepreneurship and commercialization at WashU in translating innovative research into practical technologies that benefit patient care.”
The device generates a five-year risk score intended to supplement, not replace, the assessment performed by radiologists, who will continue to scrutinize the mammograms adhering to established protocols. According to the American Society of Clinical Oncology and the U.S. Preventive Services Task Force, a five-year risk score of 3% or greater is deemed elevated. Guidelines from these organizations recommend that women with elevated scores be directed to specialists who can further counsel them on choices for additional screening and preventive measures.
About one in eight women in the U.S. will face a breast cancer diagnosis in their lifetime. Those identified as being at elevated risk have the opportunity to undergo more frequent screenings — which may include other imaging modalities, like MRI — and in certain cases, may opt for preventive treatments such as a chemotherapy agent called tamoxifen or endocrine therapy. With such alternatives available, pinpointing women at high risk is crucial, so they can access specialists who can assist them in making these significant decisions.
The developers are preparing a clinical trial at Siteman Cancer Center that will apply the risk score from Prognosia Breast alongside the standard mammography screening protocols. Standard screening protocols involve the examination of mammograms and measurements of breast density already available to all patients. Individuals classified as elevated risk will be referred to Siteman’s breast health specialists, who concentrate on assisting individuals in navigating their options for managing high breast cancer risk.
“Despite the advances in today’s breast imaging and its widespread implementation for…
“““html
“While pinpointing existing tumors, the current risk assessment for breast cancer continues to rely on surveys and is not particularly effective at predicting future danger,” stated Jiang. “Our research has concentrated on addressing the demand for improved methodologies. Transitioning to image-based risk evaluation — which our findings indicate is significantly more precise — holds the promise of being groundbreaking for patient treatment.”
The existing FDA designation pertains to the software’s evaluation of mammogram images taken at a single instance. Looking ahead, the researchers intend to enhance Prognosia Breast to examine multiple years of mammograms from the same patient, which may further elevate the prediction’s precision.
About Washington University School of Medicine
WashU Medicine is a worldwide frontrunner in academic health care, encompassing biomedical research, patient treatment, and instructional programs with 2,900 faculty members. Its National Institutes of Health (NIH) research funding portfolio ranks second among U.S. medical schools and has surged by 83% since 2016. Alongside institutional funding, WashU Medicine invests well over $1 billion each year in basic and clinical research breakthroughs and training. Its faculty practice consistently ranks within the top five nationally, with more than 1,900 faculty physicians operating across 130 sites. WashU Medicine physicians are the exclusive staff for Barnes-Jewish and St. Louis Children’s hospitals — the academic facilities of BJC HealthCare — and provide care at BJC’s community hospitals in our area. WashU Medicine has a rich legacy in MD/PhD education, having recently dedicated $100 million to scholarships and curriculum revitalization for its medical scholars, and is home to outstanding training programs across all medical specialties, as well as physical therapy, occupational therapy, and audiology and communication sciences.
Originally published on the WashU Medicine website
The post AI-based breast cancer risk technology receives FDA Breakthrough Device designation appeared first on The Source.
“`