Motivated by a hitchhiking fish that utilizes a unique suction apparatus to cling to sharks and various sea creatures, scientists from MIT and other organizations have developed a mechanical adhesive tool that can attach to soft surfaces underwater or in harsh conditions, remaining in place for days or weeks.
This tool, as demonstrated by the researchers, can adhere to the lining of the gastrointestinal tract, where the mucosal layer complicates the attachment of any kind of sensor or drug-delivery capsule. With their innovative adhesive technology, the researchers illustrated that they could achieve automatic self-adhesion, without any motors, to deliver HIV antiviral medications or RNA to the GI tract; it could also serve as a sensor for gastroesophageal reflux disease (GERD). Additionally, the device can connect to a swimming fish to observe aquatic ecosystems.
The design is grounded in the research team’s thorough investigations of the remora’s sucker-like disc. These discs possess numerous distinct qualities that enable them to attach firmly to a range of hosts, including sharks, marlins, and rays. Yet, the mechanisms by which remoras maintain adherence to soft, dynamically fluctuating surfaces remain mostly unexplored.
“Grasping the essential physics and mechanics behind how this part of the fish clings to another organism guided us in establishing the foundation for engineering a synthetic adhesive system,” states Giovanni Traverso, an associate professor of mechanical engineering at MIT, a gastroenterologist at Brigham and Women’s Hospital, an associate member of the Broad Institute of MIT and Harvard, and the lead author of the study.
MIT research scientist Ziliang (Troy) Kang serves as the primary author of the study, which is published today in Nature. The research group also consists of contributors from Brigham and Women’s Hospital, the Broad Institute, and Boston College.
Nature’s Inspiration
Most protein and RNA-based drugs cannot be administered orally because they will be degraded before absorption into the GI tract. To tackle this issue, Traverso’s lab is focusing on swallowable devices designed to release their payload gradually over days, weeks, or even longer.
A major challenge is that the digestive tract is coated with a slippery mucosal membrane that continuously regenerates, making it difficult for any device to adhere. Moreover, any device that succeeds in attaching to this lining is likely to be dislodged by food or liquids traversing the tract.
To seek solutions to these dilemmas, the MIT team turned to the remora, also referred to as the sucker fish, which clings to its hosts for free rides and access to food leftovers. To investigate how the remora adheres so strongly to dynamic, soft surfaces, Traverso collaborated with Christopher Kenaley, an associate professor of biology at Boston College, who studies remoras and other fish.
Their investigations revealed that the remora’s sticking ability relies on several distinct attributes. Initially, the substantial suction disc generates adhesion via pressure-based suction, much like a plunger. Furthermore, each disc is segmented into small adhesive compartments by rows of plates known as lamellae encased in soft tissue. These compartments can independently exert additional suction on heterogeneous soft surfaces.
There exist nine species of remora, and in each species, these lamellae rows are oriented somewhat differently—some are entirely parallel, while others exhibit patterns with rows tilted at various angles. The researchers discovered that these variations might be crucial to understanding each species’ evolutionary adaptation to its host.
Remora albescens, a distinctive species that displays mucoadhesion in the oral cavities of rays, inspired the team to create devices with improved adhesion to soft surfaces, leveraging its unique and highly tilted lamellae orientation. Conversely, other remora species, which attach to fast swimmers like marlins and swordfish, generally possess highly parallel orientations, allowing the hitchhikers to glide without losing adhesion while being swiftly pulled through the water. Still others, having a combination of parallel and angled rows, can adhere to various hosts.
Microscopic spines that emerge from the lamellae contribute to enhanced adhesion by interlocking with the host tissue. These spines, known as spinules, measure several hundred microns in length and cling to the tissue with minimal invasiveness.
“If the suction within the compartment experiences shear force, the friction caused by the mechanical interlocking of the spinules can aid in maintaining the suction,” states Kang.
Aquatic Environments
By emulating these anatomical features, the MIT team successfully created a device with robust adhesion suitable for a wide range of underwater applications.
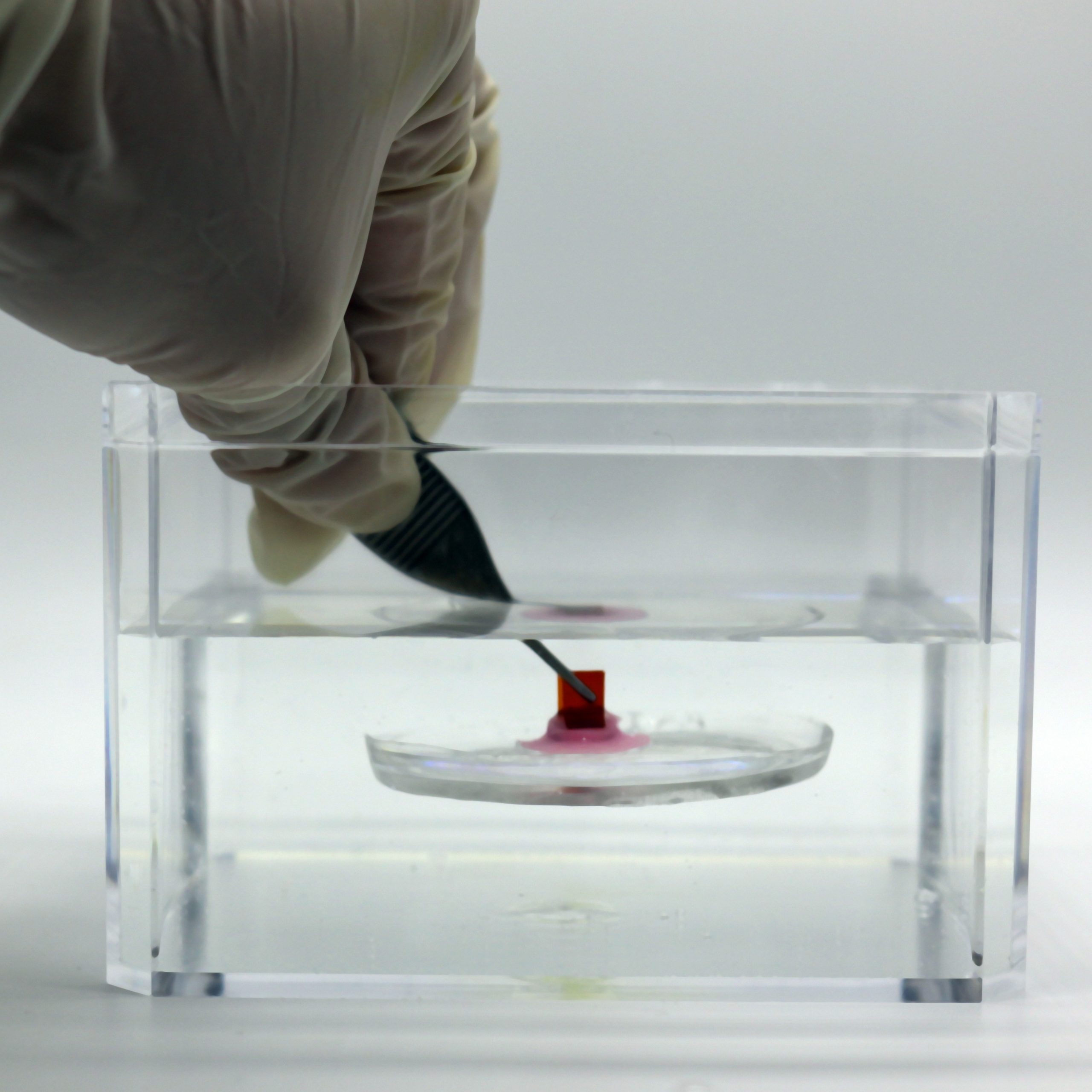
The researchers utilized silicone rubber and temperature-responsive smart materials to fabricate their adhesive device, named MUSAS (short for “mechanical underwater soft adhesion system”). The entirely passive, disc-shaped device includes rows of lamellae akin to those of the remora and can self-adhere to the mucosal lining, capitalizing on GI contractions. The researchers found that for their aims, a configuration of tilted rows was the most efficient.
Within the lamellae are minuscule microneedle-like structures that replicate the spinules found in the remora. These tiny spines are crafted from a shape memory alloy that activates upon exposure to body temperatures, allowing the spines to interlock and cling to the tissue surface.
The team demonstrated that this device could attach to a variety of soft surfaces, even in moist or highly acidic environments, including pig stomach tissue, nitrile gloves, and a tilapia swimming in a tank. Subsequently, they evaluated the device for diverse applications, such as aquatic environmental monitoring. By integrating a temperature sensor into the device, the researchers proved they could attach the device to a fish and accurately measure water temperature while the fish swam at high speeds.
To showcase medical applications, the researchers incorporated an impedance sensor into the device, demonstrating that it could adhere to the esophagus in an animal model, permitting them to monitor the reflux of gastric fluid. This could provide an alternative to current GERD sensors, which are delivered via a tube that is inserted through the nose or mouth and secured to the lower part of the esophagus.
They also demonstrated that the device could be employed for the sustained release of two distinct types of therapeutics in animal tests. Initially, they showcased the integration of an HIV drug named cabotegravir into the materials that comprise the device (polycaprolactone and silicone). Once affixed to the stomach lining, the drug gradually diffused from the device over the course of a week.
Cabotegravir is one of the medications used for HIV PrEP — pre-exposure prophylaxis — as well as treatment of HIV. These treatments are typically administered as a daily pill or an injection given every one to two months.
Additionally, the researchers devised a variant of the device for delivering larger molecules such as RNA. For this delivery method, they incorporated RNA into the microneedles of the lamellae, which could then inject it into the stomach lining. Utilizing RNA that encodes the gene for luciferase, a protein that emits light, they established that they could successfully deliver the gene to cells in the cheek or esophagus.
The researchers now intend to adapt the device for the delivery of other types of medications as well as vaccines. Another potential application includes using the devices for electrical stimulation, which Traverso’s lab has previously demonstrated can activate hormones that regulate appetite.
The research received partial funding from the Gates Foundation, MIT’s Department of Mechanical Engineering, Brigham and Women’s Hospital, and the Advanced Research Projects Agency for Health.