“`html
Initially perceived solely as insulating, a change in the orientation between silicon and oxygen atoms forms a pathway for electrical charge
A newly identified silicone variant is a semiconductor, as discovered by researchers from the University of Michigan—challenging previous beliefs that this material category is exclusively insulating.
“This material paves the way for the development of new kinds of flat panel displays, flexible photovoltaics, wearable sensors, or even textiles that can exhibit various patterns or images,” stated Richard Laine, U-M professor of materials science and engineering as well as macromolecular science and engineering, and the corresponding author of the research recently published in Macromolecular Rapid Communications.
Silicone oils and rubbers—polysiloxanes and silsesquioxanes—are conventionally insulating materials, meaning they obstruct the movement of electricity or heat. Their water-resistant characteristics render them beneficial in biomedical devices, sealants, electronic coatings, and beyond.
In contrast, traditional semiconductors are characteristically rigid. Semiconducting silicone has the potential to facilitate the flexible electronics Laine described, along with silicone that comes in a palette of colors.
On the molecular scale, silicones comprise a backbone of alternating silicon and oxygen atoms (Si—O—Si) with organic (carbon-based) groups attached to the silicon. Various 3D configurations of polymer chains emerge as they bond with each other, a process known as cross-linking, which modifies the material’s physical attributes like strength or solubility.
While investigating different cross-linking frameworks in silicone, the research team unexpectedly discovered the potential for electrical conductivity in a copolymer, which is a polymer chain made up of two different types of repeating units—cage-structured and linear silicones in this situation.
The potential for conductivity stems from the manner in which electrons can traverse Si—O—Si bonds with overlapping orbitals. Semiconductors possess two primary states: the ground state, which does not conduct electricity, and a conducting state, which does. The conducting state, oft referred to as an excited state, arises when certain electrons leap to the subsequent electron orbital, forming connections throughout the material akin to that of a metal.
Typically, Si—O—Si bond angles do not permit such a connection. At 110°, they diverge significantly from a 180° straight line. However, in the silicone copolymer the team discovered, these bonds began at 140° in the ground state—and extend to 150° in the excited state. This alteration was sufficient to create a pathway for electrical charge flow.
“This facilitates an unpredicted interaction between electrons across multiple bonds, including Si—O—Si bonds in these copolymers,” Laine articulated. “The greater the chain length, the simpler it becomes for electrons to traverse longer distances, subsequently decreasing the energy required to absorb light and then release it at lower energies.”
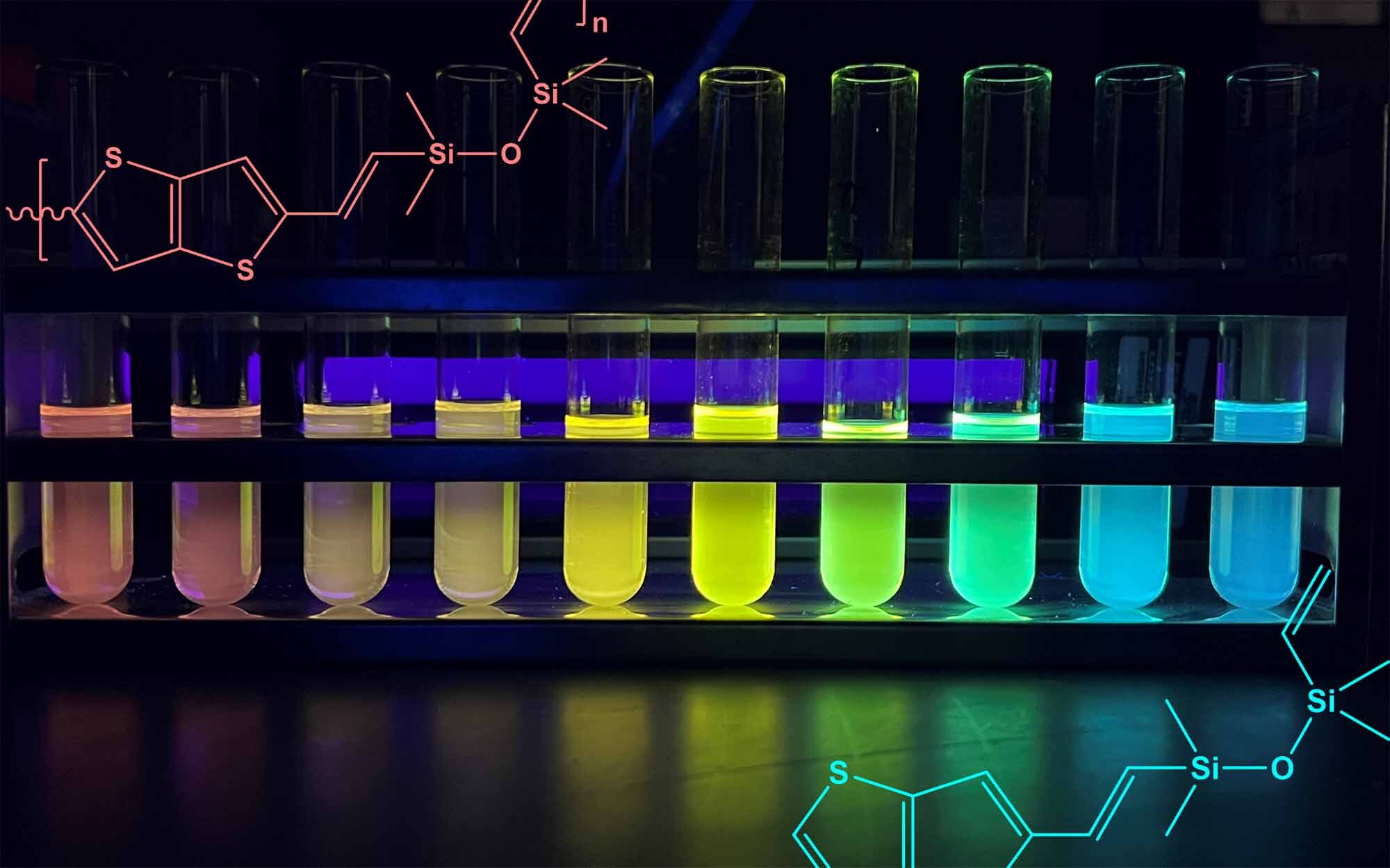
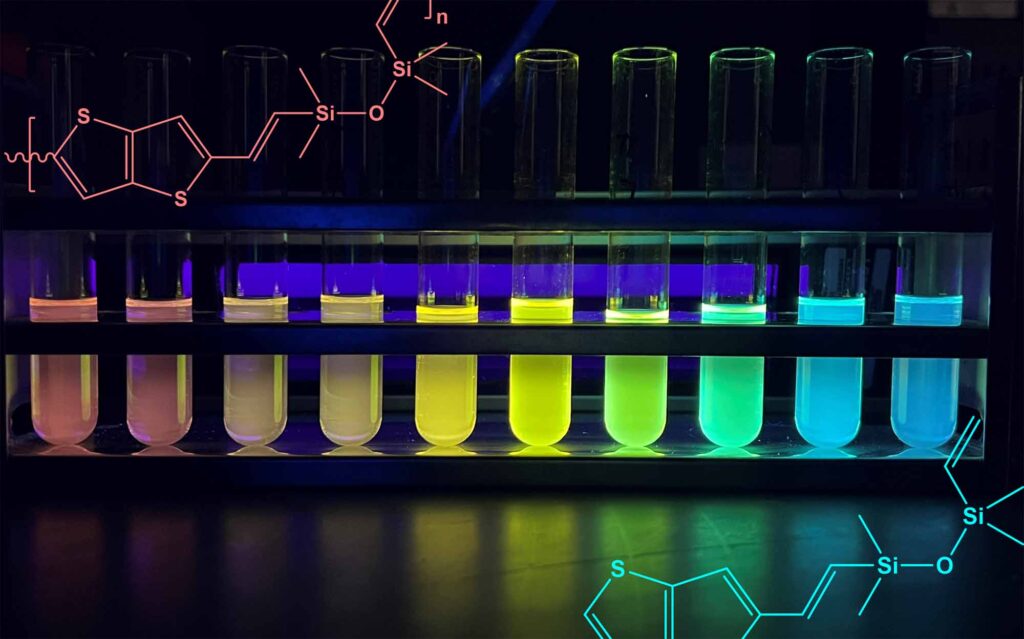
The semiconducting characteristics of the silicone copolymers also allow for their distinctive range of colors. Electrons transition between the ground and excited states by absorbing and emitting photons, or light particles. The emission of light relies on the copolymer chain’s length, which Laine’s team can manipulate. Extended chain lengths result in smaller jumps and lower energy photons, imparting a red hue to the silicone. Conversely, shorter chains necessitate larger jumps from the electrons, thereby emitting higher energy light towards the blue end of the spectrum.
To showcase the correlation between chain length and light absorption and emission, the researchers segregated copolymers of various chain lengths and organized them in test tubes from long to short. Shining a UV light on the tubes creates a vibrant rainbow as each absorbs and emits light at distinct energies.
The colorful arrangement based on copolymer chain length is especially remarkable because, until now, silicones were known to be only transparent or white due to their insulating qualities preventing them from absorbing much light.
“We are transforming a material that was once thought to be electrically inert and breathing new life into it—potentially powering the next generation of soft, flexible electronics,” remarked Zijing (Jackie) Zhang, U-M doctoral student of materials science and engineering and lead author of the study.
The research received funding from the U.S. National Science Foundation (2103628) and the Thailand National Science, Research and Innovation Fund (NSRF) via the Program Management Unit for Human Resources & Institutional Development, Research and Innovation (B16F640099).
“`