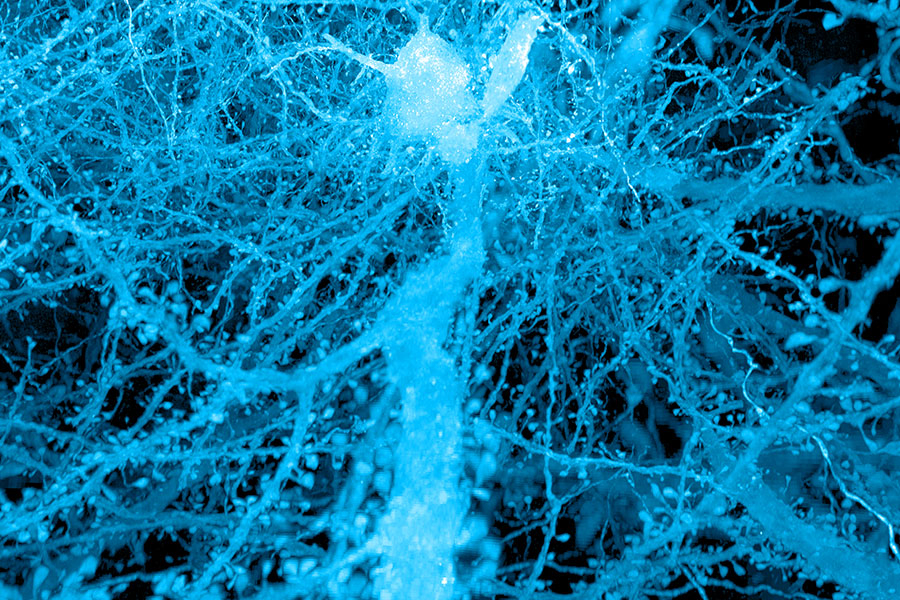
Almost 150 years prior, researchers began to envision how data could traverse the brain, inspired by the forms of neurons visible through the microscopes of their era. With contemporary imaging methods, scientists can delve much deeper, observing the minuscule synapses that allow neurons to interact and even the molecules employed by cells to transmit their signals. These internal perspectives can ignite fresh concepts regarding the functionality of healthy brains and highlight crucial transformations that lead to illness.
This enhanced understanding of biology is not solely due to advancements that have rendered microscopes more powerful than ever. Utilizing techniques pioneered in the laboratory of MIT McGovern Institute for Brain Research investigator Edward Boyden, researchers globally are imaging samples that have been inflated to as much as 20 times their original dimensions, allowing for a clearer observation of their finest characteristics.
“This represents a very different approach to microscopy,” states Boyden, who is also an investigator at the Howard Hughes Medical Institute (HHMI), a professor of brain and cognitive sciences and biological engineering, and a member of the Yang Tan Collective at MIT. “Unlike the last 300 years of bioimaging, which utilized a lens to amplify light from an object, we amplify the objects themselves physically.” Once a tissue is enlarged, Boyden explains that researchers can achieve clearer views even with commonly available conventional microscopy equipment.
In 2015, Boyden’s team introduced this methodology, which they coined expansion microscopy (ExM). Since then, they have been perfecting the technique and enhancing its functionalities, while researchers at MIT and across the globe utilize it to investigate life at the most minute scales.
“It’s rapidly proliferating throughout the fields of biology and medicine,” says Boyden. “It’s being used for kidney disorders, the brain of fruit flies, plant seeds, the microbiome, Alzheimer’s disease, viruses, and much more.”
Origins of ExM
In creating expansion microscopy, Boyden and his team looked towards hydrogel, a substance with extraordinary water-absorbing capabilities that had already proven useful; it is layered within disposable diapers to ensure infants stay dry. Boyden’s laboratory theorized that hydrogels could maintain their structure while absorbing hundreds of times their original weight in water, thereby expanding the gaps between their chemical constituents as they swell.
After conducting a series of experiments, Boyden’s team determined four essential steps for enlarging tissue samples to enhance imaging. First, the tissue must be saturated with a hydrogel. The tissue’s components, biomolecules, are tethered to the gel’s web-like structure, directly linking them to the molecules comprising the gel. Next, the tissue undergoes chemical softening, along with the addition of water. As the hydrogel takes in the water, it swells, causing the tissue to expand uniformly while preserving the relative arrangement of its components.
Boyden and graduate students Fei Chen and Paul Tillberg’s initial report on expansion microscopy was published in the journal Science in 2015. In this publication, the team demonstrated that by spacing apart molecules that had previously been cramped within cells, features that would have fused together under a typical light microscope became distinct and separate. Conventional light microscopes can differentiate between objects separated by about 300 nanometers—a limitation dictated by the laws of physics. Through expansion microscopy, Boyden’s team reported an effective resolution of around 70 nanometers, achieved through fourfold expansion.
Boyden notes that this level of detail is essential for biologists. “Biology, in essence, operates at the nanoscale,” says Boyden. “Biomolecules exist at the nanoscale, and their interactions occur over nanoscale distances. Many critical questions in biology and medicine pertain to nanoscale issues.” Various types of advanced microscopes, each possessing unique advantages and drawbacks, are capable of illuminating such fine details. However, these methodologies are often expensive and demand specialized expertise, rendering them inaccessible for the majority of researchers. “Expansion microscopy democratizes nanoimaging,” asserts Boyden. “Now, anyone can explore the fundamental building blocks of life and their interrelations.”
Empowering scientists
Since its introduction in 2015, Boyden’s team’s expansion microscopy has empowered research groups worldwide, resulting in hundreds of publications detailing discoveries made using this technique. For neuroscientists, this method has illuminated the complexities of intricate neural networks, revealed how specific proteins arrange themselves at synapses to facilitate neuron communication, and uncovered alterations linked to aging and disease.
This technique has also proven invaluable for studies extending beyond the brain. Sabrina Absalon employs expansion microscopy weekly in her laboratory at Indiana University School of Medicine to investigate the malaria parasite, a single-celled organism enriched with specialized structures that enable it to infect and survive within hosts. The parasite is diminutive, with most of its structures being indiscernible through standard light microscopy. “As a cell biologist, I’m forfeiting the primary tool for inferring protein function, organelle architecture, morphology related to function, and all of those aspects — which is my eye,” she elaborates. With expansion microscopy, not only can she visualize the organelles within a malaria parasite, but she can also observe their assembly and monitor their behavior during the parasite’s division. Gaining insight into those processes, she states, could assist drug developers in discovering new methods to disrupt the parasite’s life cycle.
Absalon emphasizes that the accessibility of expansion microscopy holds particular significance in parasitology, where substantial research is conducted in regions with limited resources. Workshops and training initiatives in Africa, South America, and Asia are ensuring that the technology reaches scientists whose communities are directly affected by malaria and other parasites. “Now they can obtain super-resolution imaging without requiring highly sophisticated equipment,” Absalon notes.
Always improving
Since 2015, Boyden’s interdisciplinary lab has discovered numerous inventive ways to enhance expansion microscopy and apply it creatively. Their current standard technique offers improved labeling, larger expansion factors, and higher-resolution imaging. Cellular features that are less than 20 nanometers apart can now be sufficiently separated to appear distinctive under a light microscope.
They’ve also modified their protocols to accommodate a variety of significant sample types, ranging from entire roundworms (popular among neuroscientists, developmental biologists, and other experts) to clinical samples. In this context, they’ve demonstrated that expansion can unveil subtle indications of disease, enabling earlier or more economical diagnoses.
Initially, the group tailored its protocol to visualize proteins within cells by labeling proteins of interest and anchoring them to the hydrogel prior to expansion. With a newly developed sample processing technique, users can now re-stain their expanded samples with different labels for multiple imaging rounds, allowing them to locate the positions of dozens of different proteins within the same tissue. This capability enables researchers to visualize how molecules are arranged concerning one another and how they may interact, or to survey extensive protein sets to ascertain what changes, for instance, occur during disease.
However, elucidating proteins was merely the beginning for expansion microscopy. “We aspire to visualize everything,” Boyden declares. “We would love to observe every biomolecule there is, with precision down to the atomic scale.” They are not there yet — but with new probes and revised methods, achieving this goal is now within reach.
see not only proteins, but also RNA and lipids in amplified tissue samples.
Labeling lipids, which include those that create the membranes encasing cells, enables researchers to observe distinct outlines of cells in expanded tissues. With the improved clarity provided by expansion, even the fine extensions of neurons can be traced through a visual representation. Historically, scientists have depended on electron microscopy, which produces remarkably intricate images but necessitates costly equipment, to chart the brain’s circuitry. “Now, you can acquire images that resemble electron microscopy images significantly, but using standard light microscopes — the sort that everyone can access,” Boyden states.
Boyden remarks that expansion can be incredibly effective when paired with other state-of-the-art tools. When expanded samples are applied alongside an ultra-rapid imaging technique developed by Eric Betzig, an HHMI investigator at the University of California at Berkeley, known as lattice light-sheet microscopy, the complete brain of a fruit fly can be imaged at high fidelity in just a few days.
Moreover, when RNA molecules are embedded within a hydrogel matrix and subsequently sequenced on-site, researchers can pinpoint exactly where within cells the directives for constructing specific proteins are located, which Boyden’s team illustrated in collaboration with Harvard University geneticist George Church and then-MIT professor Aviv Regev. “Expansion essentially enhances the resolution of many other technologies,” Boyden explains. “Are you conducting mass-spec imaging, X-ray imaging, or Raman imaging? Expansion just elevated your instrument’s performance.”
Broadening possibilities
A decade after the initial demonstration of expansion microscopy’s capabilities, Boyden and his team remain dedicated to enhancing the power of expansion microscopy. “We aim to refine it for various challenges, and making technologies quicker, more efficient, and less costly is always crucial,” he states. However, the future of expansion microscopy will also be driven by innovators beyond the Boyden laboratory. “Expansion is not only straightforward to implement; it’s also adaptable — many other researchers are enhancing expansion in partnership with us, or even independently,” Boyden remarks.
Boyden highlights a team led by Silvio Rizzoli at the University Medical Center Göttingen in Germany that, in collaboration with Boyden, has modified the expansion protocol to identify the physical configurations of proteins. At the Korea Advanced Institute of Science and Technology, researchers directed by Jae-Byum Chang, a former postdoctoral researcher in Boyden’s group, have discovered how to expand entire bodies of mouse embryos and juvenile zebrafish, working with Boyden to pave the way for investigating developmental processes and long-range neural connections with a novel level of detail. Additionally, mapping connections within the brain’s intricate neural networks may become more manageable with light-microscopy-based connectomics, a method formulated by Johann Danzl and colleagues at the Institute of Science and Technology in Austria, leveraging both the high resolution and molecular details that expansion microscopy can unveil.
“The elegance of expansion lies in its ability to allow you to visualize a biological system down to its most fundamental components,” Boyden asserts.
His team is focused on pushing the technique to its physical limits and foresees new avenues for exploration as they proceed. “If you can chart the brain or any biological system at the level of individual molecules, you may be able to understand how they all collaborate as a network — how life truly functions,” he concludes.