“`html
In 2015, Andre Hoelz, the Mary and Charles Ferkel Professor of Chemistry and Biochemistry and a Howard Hughes Medical Institute researcher, discovered that he had an uncommon brain tumor pressing against his auditory nerve. The chemist, who typically concentrates on elucidating the structure of protein complexes in his laboratory, embarked on a journey to acquire as much knowledge as possible about the mechanics of the auditory system.
Eventually, he collaborated with his surgeon, Rick Friedman, who is also a research scientist and vice chair at UC San Diego with expertise in ear disorders. Together, they have undertaken the quest to find strategies for safeguarding the inner ear from hearing impairment. Now, Hoelz and Friedman have secured funding to broaden their experimental agenda.
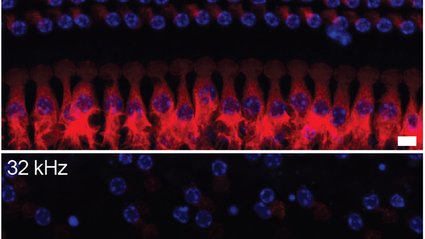
The inner ear is a remarkable yet somewhat delicate mechanism for transmitting sound from the outer ear to the brain. It accomplishes this through tiny clusters of hair-like cells that react to incoming sound waves by releasing neurotransmitters, which send auditory information to the brain along the auditory nerve. These inner ear hair cells are essential for our ability to hear. Unfortunately, they cannot be regenerated. Once they are lost, they are permanently gone, and the hearing they facilitated vanishes with them.
“Certain cells in our body are so specialized that they cannot be replenished,” Hoelz clarifies. “The cells responsible for our hearing, along with other sensory cells, fall into this category. We possess 37 trillion cells in our body, and merely 16,000 of them are involved in hearing. When these cells perish, our hearing is lost. And concerning inner ear hair cells, it’s not a question of if they will perish, but rather when.”
Inner ear hair cells (IHCs) can die for numerous reasons, including viral infections, exposure to high noise levels, and natural aging processes. A particularly damaging source of hearing loss is linked to cisplatin chemotherapy, a potent and demanding cancer treatment used for adult-onset cancers (such as testicular, head and neck, ovarian, cervical, endometrial, and lung) and pediatric cancers (like neuroblastoma, osteosarcoma, medulloblastoma, retinoblastoma, and Wilms tumor).
“Especially for pediatric patients, the aim is to eliminate as many cancer cells as possible. Children potentially have another 80 years ahead of them, we hope. Thus, treatments for childhood cancers tend to be aggressive,” Hoelz explains. “Sadly, cisplatin chemotherapy can inflict serious harm on the hair cells necessary for hearing. A staggering 70% of patients treated with cisplatin ultimately experience some level of hearing loss.”
“There are no ideal solutions to counteract this toxicity,” Friedman adds.
Hearing loss is particularly detrimental for children who are in the nascent stages of developing speech and communication abilities. Around 2,000 children in the United States are subjected to cisplatin each year. The only treatment currently sanctioned by the Food and Drug Administration to prevent hearing loss in patients undergoing cisplatin therapy has a significant drawback: it neutralizes cisplatin, consequently diminishing the efficacy of the chemotherapy. “This medication is essentially an antidote for cisplatin,” Hoelz remarks.
Cisplatin therapy saves lives that would otherwise be lost to cancer. When faced with the choice of losing one’s hearing or facing death, there is often little discussion.
Hoelz himself encountered a similar choice in 2015 when he suffered from severe vertigo and temporary hearing loss. Medical professionals found that a rare brain tumor was applying pressure on his auditory nerve, impacting his balance and hearing. As Hoelz’s symptoms fluctuated, he and his healthcare team opted to regularly monitor the tumor through MRI scans and periodically assess his hearing. During this period, Hoelz began consultations with Dr. Friedman. “I had a strong sense that eventually, I would require surgery, and that Rick was the right person to conduct it,” Hoelz recalls. “However, Rick indicated that I might not be the best candidate for preserving my ear, prompting me to hesitate. I pondered, ‘I may not be fortunate, but if not, at least I can retain my hearing for a while.’
As Friedman tracked Hoelz’s tumor, the two began to engage in scientific discussions. “It was peculiar,” Hoelz comments. “I was weighing the decision to undergo surgery, but concurrently, I was captivated by Rick’s data regarding protein complexes involved in hearing, which led me to consider how my work in the lab might illuminate these processes.”
When Hoelz’s symptoms worsened considerably, he chose to go through with the surgery and lost hearing in his left ear. Yet, simultaneously, he initiated a collaboration with Friedman that has already yielded encouraging results.
Friedman states, “Meeting and collaborating with Andre is the most significant event of my scientific career.”
Over the past five years, Hoelz, Friedman, and other researchers in the field have pinpointed a gene that makes individuals more vulnerable to hearing loss—whether it arises from noise, cisplatin, or aging—and begun investigating methods to strengthen inner ear hair cells to protect hearing.
Hoelz and Friedman initially identified a gene, Prkag2, which encodes a component of the AMPK (5′ adenosine monophosphate-activated protein kinase) complex. AMPK is an enzyme functioning throughout the body, from the liver to the brain to skeletal muscle, regulating metabolism. IHCs are highly metabolically active. The synapses connecting IHCs to the auditory nerve depend on AMPK to deliver neurotransmitters—in this case, glutamate—when they are activated by sound. Without these transfers from the AMPK complex, IHCs cannot convey auditory information to the brain.
Hoelz and Friedman have demonstrated that they can indeed safeguard IHCs, and the hearing that depends on them, if an AMPK-activating drug is appropriately administered prior to cisplatin treatment. With a grant from Curebound, a charitable organization in San Diego funding cancer research, they are now determining the optimal dosage and timing needed to preserve the hearing of cisplatin-treated cancer patients. Efficacy is being evaluated in murine models. Once an ideal therapy is established, it will be tested in guinea pigs, whose auditory systems resemble our own, and finally in humans.
“The aspiration is that recruiting participants for a study of these treatments will be relatively straightforward,” Hoelz remarks. “If you are receiving cisplatin treatment, you may lose your hearing regardless, so any opportunity to retain at least some hearing should be appealing.”
The potential for this therapy does not end there. “We hope that in the long term, this could serve as a kind of ear vitamin that would be beneficial for everyone. The treatment would strengthen IHCs and make them less vulnerable to various types of damage, not just that caused by cisplatin. Much like applying a veneer to teeth to protect against cavities, this therapy could enhance the lifespan of IHCs, potentially enabling them to endure throughout a person’s life,” Hoelz states.
“While mammals cannot regenerate IHCs, certain fish and birds have that ability,” Hoelz explains. “Somehow during our evolution, we lost this capability. Scientists are investigating what developmental program might allow humans to regenerate IHCs,” he continues. “In the meantime, developing a therapy to protect them would be fantastic. Collaborating with Rick has been an exhilarating plunge into the realm of hair cell biology, opening new scientific avenues and igniting a personal hope that this research might one day aid in preserving hearing—including my own.”
“`