Achieving 98% efficiency in solid form and 94% in solution, the design of this small fluorescent molecule could significantly reduce development time and expenses for upcoming implementations
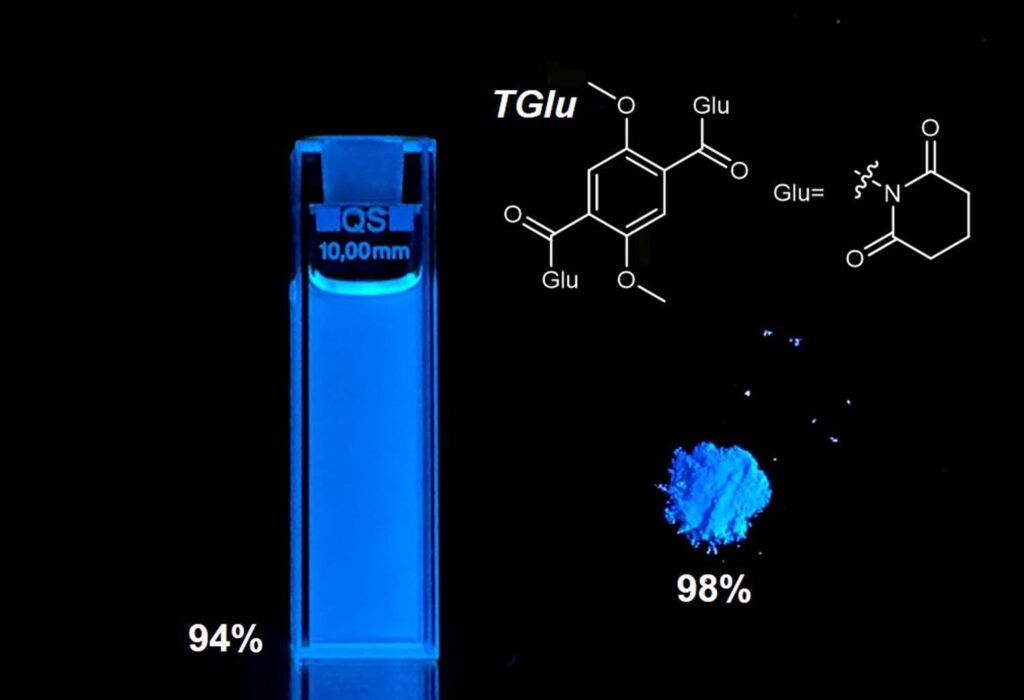
A groundbreaking blue fluorescent molecule has set new records for emission efficiency in both solid and liquid states, as indicated by a study led by the University of Michigan, which could open new avenues for technology and medical uses.
These fluorescent molecules, known as fluorophores, are capable of absorbing light and emitting it at lower energy frequencies, illuminating OLED screens and aiding researchers and physicians in understanding cellular and tissue processes. While solid forms are essential in displays and various sensing technologies, liquids tend to be more suitable for biological applications. Most fluorophores struggle to perform effectively in both states, but this one excels.
“The fluorescent material achieved record levels of brightness and efficiency, boasting 98% quantum efficiency in solid form and 94% in liquid,” stated Jinsang Kim, the Raoul Kopelman Collegiate Professor of Science and Engineering within the U-M Department of Materials Science and Engineering and the study’s leader, as published in Nature Communications.
Typically, engineers working on fluorophores initiate their processes in solution, examining the optical characteristics of separate molecules. However, they face challenges when these fluorophore molecules are packed together in solid-state applications.
“In the solid state, fluorophores exhibit markedly different behaviors, necessitating more deliberate molecular engineering efforts for structural alterations,” Kim commented. “Through the investigation and establishment of a molecular design principle to create fluorophores that shine brightly in both solution and solid states, we have diminished development timelines and costs for a variety of future applications.”
The initial identification of this multifunctional fluorophore—termed TGlu—was an unforeseen result for lead author Jung-Moo Heo, a U-M postdoctoral research fellow in materials science and engineering.
“TGlu was initially intended as an intermediary for another chemical design, but during purification, I found it to be surprisingly high in emission not only in solution but also in solid form,” Heo noted.
This discovery sparked a methodical investigation to define the ideal design. The outcome was a straightforward design: a core composed of a single benzene ring—six carbon atoms interconnected in a hexagon. The researchers arranged two electron-donating groups, known as donor groups, on opposite sides of the ring. Flanking the donors, they positioned two acceptor groups, which draw in electrons, across from one another.
“This so-called quadrupolar configuration symmetrically distributes charge throughout the molecule, yielding stable emission across diverse environments,” Heo explained.
Due to the ring’s six points, the donor and acceptor groups are located adjacent to each other. This spatial arrangement minimizes the energy gap compared to similar molecules, allowing the fluorophore to require a relatively low energy quantity to transition an electron from the ground state to an excited state—akin to climbing to a higher rung on a ladder.
Nevertheless, the molecule’s diminutive size limits the overall conjugation length—meaning electrons cannot disseminate extensively across the molecule. This constraint keeps the absolute energy gap—the distance between ladder rungs—sufficiently wide to emit blue light rather than gravitating towards colors with narrower energy gaps, such as red.
Generally, smaller band gaps come with a drawback in efficiency. When in the excited state on a higher rung, an electron has the choice to emit light as it returns to the ground state or release energy as heat through vibration. Usually, small band gaps lead to increased heat dissipation, lowering the quantum yield—an efficiency metric defined as the percentage of absorbed UV light that gets re-emitted as visible light relative to the energy lost as heat.
After testing various acceptor groups, the researchers discovered one that stabilizes the excited state. Even with a small band gap, this group inhibits heat loss by limiting access to what are termed conical intersections, which act as “exit points” for energy leakage. This unanticipated behavior, termed an Inverted Energy Gap Law, was validated through both experimental and quantum chemical simulations.
In the solid state, the intentionally bulky acceptor groups prevent the molecules from becoming too close, a condition that can diminish brightness as energy escapes as heat rather than light, a phenomenon known as quenching.
This small, highly efficient fluorophore is straightforward to manufacture—only needing three steps—thus enhancing its scalability while minimizing production costs.
The existing TGlu structure fluoresces blue light. Moving forward, researchers intend to adjust the band gap to alter the color. Additionally, while a high quantum yield from light excitation is encouraging, evaluating device performance under electrical excitation will require further investigation due to other loss mechanisms. Heo also aims to develop a phosphorescent variant of the molecule, as phosphors are generally more energy-efficient than fluorophores for application in display technologies.
The Autonomous University of Madrid, University of Valencia, Eberhard Karls University Tübingen, and Seoul National University also played a role in this research.
