“`html
Utilizing a cost-effective electrode coated with DNA, researchers from MIT have developed disposable diagnostics that could be tailored to identify numerous ailments, including cancer and infectious diseases like influenza and HIV.
These electrochemical sensors utilize a DNA-cleaving enzyme associated with the CRISPR gene-editing technology. When a target, such as a malignant gene, is identified by the enzyme, it commences an indiscriminate cleavage of DNA from the electrode, akin to a lawnmower trimming grass—thereby modifying the electrical signal generated.
A significant drawback of this sensing technology is that the DNA covering the electrode degrades rapidly, which means the sensors cannot be preserved for extended periods, and must be kept under specific conditions, thus restricting their usability. In a recent study, MIT researchers enhanced the DNA’s stability with a polymer coating, enabling the sensors to be stored for up to two months, even in elevated temperatures. After this storage period, the sensors successfully detected a prostate cancer gene, commonly used for disease diagnosis.
The DNA-based sensors, which can be produced for around 50 cents each, may provide a more affordable means of diagnosing numerous conditions in resource-limited areas, according to Ariel Furst, the Paul M. Cook Career Development Assistant Professor of Chemical Engineering at MIT and the principal author of the study.
“Our emphasis is on diagnostics that are often inaccessible to many people, and our objective is to create a point-of-use sensor. Individuals wouldn’t even need to be in a clinic to utilize it; they could do so at home,” Furst asserts.
MIT graduate student Xingcheng Zhou is the lead author of the publication, released on June 30 in the journal ACS Sensors. Additional authors include MIT undergraduate Jessica Slaughter, Smah Riki ’24, and graduate student Chao Chi Kuo.
A budget-friendly sensor
Electrochemical sensors function by assessing variations in the flow of electric current when a target molecule interacts with an enzyme. This principle is utilized by glucose meters to determine glucose levels in blood samples.
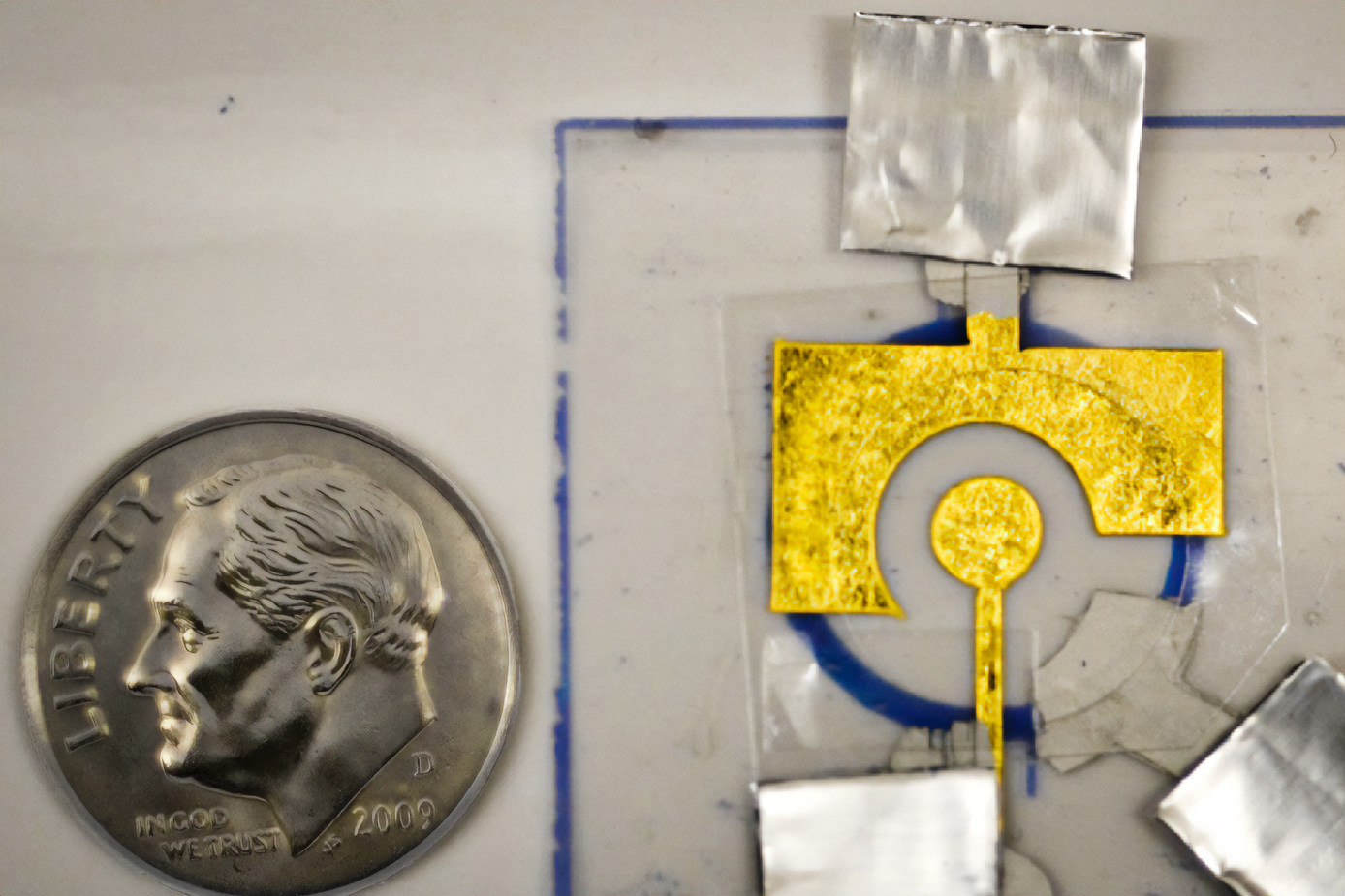
The electrochemical sensors produced in Furst’s laboratory comprise DNA affixed to a low-cost gold leaf electrode, which is bonded to a sheet of plastic. The DNA is attached to the electrode via a sulfur-containing compound known as a thiol.
In a study conducted in 2021, Furst’s lab demonstrated that these sensors could detect genetic material from HIV and human papillomavirus (HPV). The sensors identify their targets using a guide RNA strand, designed to bind to nearly any sequence of DNA or RNA. This guide RNA is coupled with an enzyme called Cas12, which nonspecifically cleaves DNA when activated and belongs to the same protein family as the Cas9 enzyme used for CRISPR genome editing.
If the target is present, it associates with the guide RNA and activates Cas12, which then cleaves the DNA attached to the electrode. This alters the current produced by the electrode, measurable by a potentiostat (the same technology found in handheld glucose meters).
“When Cas12 is activated, it operates like a lawnmower that clears away all the DNA on your electrode, which subsequently shuts off your signal,” Furst explains.
In earlier iterations of the device, DNA had to be added to the electrode just prior to usage, as it doesn’t remain stable for long. In the current study, researchers discovered they could enhance the stability of the DNA by coating it with a polymer named polyvinyl alcohol (PVA).
This polymer, which costs under 1 cent per coat, acts like a tarp to shield the underlying DNA. Once applied to the electrode, the polymer dries to form a protective thin layer.
“After it dries, it appears to create a robust barrier against the primary agents that can damage DNA, such as reactive oxygen species that can either degrade the DNA itself or sever the thiol bond with the gold, causing your DNA to detach from the electrode,” Furst notes.
Effective detection
The researchers demonstrated that this coating could preserve DNA on the sensors for a minimum of two months and could endure temperatures of up to approximately 150 degrees Fahrenheit. After two months, they removed the polymer and showed that the sensors could still identify PCA3, a prostate cancer gene detectable in urine.
This type of testing could be applicable to various samples, including urine, saliva, or nasal swabs. The researchers aspire to utilize this method to create more affordable diagnostics for infectious diseases, such as HPV or HIV, suitable for use in doctors’ offices or at home. This approach could also be expanded to develop tests for emerging infectious diseases, according to the researchers.
A team from Furst’s lab has recently gained acceptance into delta v, MIT’s student venture accelerator, where they aim to establish a startup to further advance this technology. With the ability to create tests that have a significantly longer shelf life, they intend to start distributing them to locations where they can be tested with patient samples.
“Our aim is to continue conducting tests with patient samples across various diseases in real-world conditions,” Furst states. “Our previous limitation was that we had to manufacture the sensors on-site, but now that we have a way to protect them, we can ship them. Refrigeration isn’t necessary. This expands our access to many more rugged or non-ideal testing environments.”
This research was partially funded by the MIT Research Support Committee and a MathWorks Fellowship.
“`