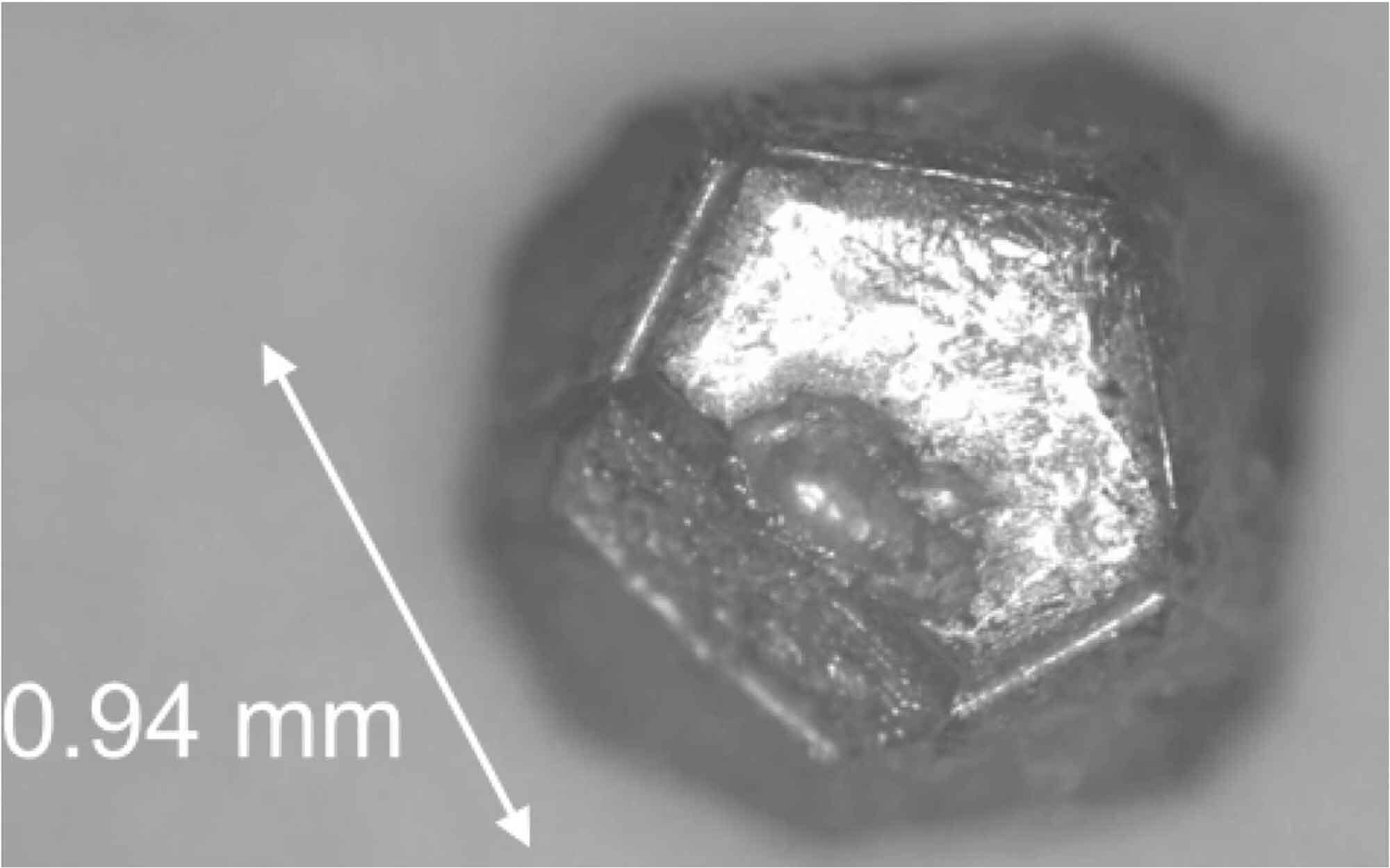
“`html
Quasicrystals could not be replicated using quantum mechanics due to their nonuniform atomic arrangements. A novel technique addresses this difficulty.
An uncommon and intriguing intermediate state between crystals and glass can be the most stable configuration for certain atomic combinations, as indicated by research from the University of Michigan.
The results stem from the inaugural quantum-mechanical simulations of quasicrystals—a kind of solid that researchers previously believed could not materialize. Although the atoms in quasicrystals form a lattice, similar to a crystal, their atomic arrangements do not repeat as seen in traditional crystals. The new simulation technique implies that quasicrystals—akin to crystals—are intrinsically stable substances, notwithstanding their resemblance to disordered solids like glass that arise from rapid heating and cooling.

“We must understand how to position atoms into specific formations if we aim to create materials with specific characteristics,” stated Wenhao Sun, the Dow Early Career Assistant Professor of Materials Science and Engineering and the main author of the article published today in Nature Physics. “Quasicrystals have compelled us to reconsider how and why certain materials can develop. Prior to our investigation, it was uncertain to scientists why they existed.”
Quasicrystals appeared to contradict physical laws when they were first introduced by the Israeli scientist Daniel Shechtman in 1984. While experimenting with aluminum and manganese alloys, Shechtman discovered that certain metal atoms were arranged in an icosahedral arrangement, resembling multiple 20-sided dice joined at their planes. This formation endowed the material with five-fold symmetry—identical from five distinct angles.
Researchers at that time believed that the atoms within crystals could only arrange themselves in sequences that repeat uniformly in every direction, yet five-fold symmetry excluded such arrangements. Shechtman initially faced significant scrutiny for positing the unfeasible, but additional laboratories subsequently produced their own quasicrystals and discovered them in ancient meteorites.
Shechtman ultimately received the Nobel Prize in Chemistry in 2011 for his revelation, but fundamental questions regarding the formation of quasicrystals remained unanswered. The obstacle was that density functional theory—the quantum-mechanical approach for assessing a crystal’s stability—depends on patterns that infinitely cycle in a sequence, which quasicrystals lack.

“The initial step to comprehending a material is understanding what makes it stable, but it has been challenging to discern how quasicrystals were stabilized,” remarked Woohyeon Baek, a U-M doctoral student in materials science and engineering and the lead author of the study.
Typically, the atoms in any material organize into crystals to allow chemical bonds to attain the lowest energy state possible. Such configurations are referred to as enthalpy-stabilized crystals. However, other materials arise from high entropy, indicating there are numerous various ways for its atoms to arrange or oscillate.
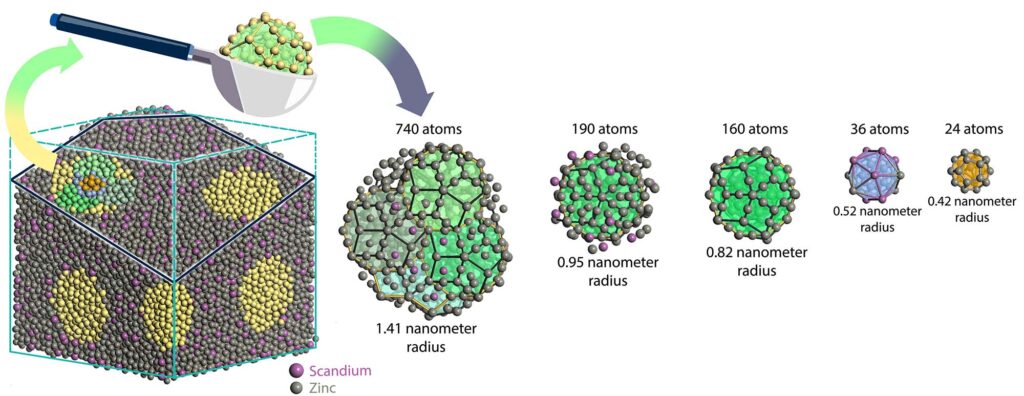
“““html
don’t reiterate in a sequence, the scholars modeled samples of quasicrystal that were randomly extracted from a larger mass. The energy within each nanoparticle can be determined utilizing quantum mechanics since the particle has defined limits. Repeating these evaluations across various scoop dimensions enables the scholars to extend their energy computations to the bulk quasicrystal. Image credit: Woohyeon Baek, Sun Research Group, University of Michigan
Glass serves as an example of an entropy-stabilized solid. It forms when melted silica rapidly cools, flash-freezing the atoms into an unpatterned structure. However, if the cooling rates decrease, or a base is introduced to heated silica, the atoms may organize into quartz crystals—the preferred, minimal energy state at room temperature. Quasicrystals represent a perplexing midpoint between glass and crystal. They exhibit locally ordered atomic configurations akin to crystals, but, similar to glass, they do not establish long-range, repetitive patterns.

To ascertain whether quasicrystals are enthalpy- or entropy-stabilized, the researchers’ approach extracts smaller nanoparticles from a larger simulated quasicrystal block. They then compute the total energy contained within each nanoparticle, which does not necessitate an infinite sequence due to the definitive boundaries of the particle.
Since the energy in a nanoparticle correlates with its volume and surface area, repeating the computations for nanoparticles of increasing sizes allows the researchers to project the total energy contained within a larger block of quasicrystal. Through this process, it was discovered that two extensively studied quasicrystals are enthalpy-stabilized. One contains an alloy of scandium and zinc, while the other comprises ytterbium and cadmium.
The most precise assessments of quasicrystal energy require the largest possible particles; however, enlarging the nanoparticles presents challenges with conventional algorithms. For nanoparticles containing only hundreds of atoms, doubling the atom count results in an eightfold increase in computing time. Nevertheless, the researchers identified a solution for this computational challenge as well.
“In traditional algorithms, each computer processor must communicate with others, whereas our algorithm operates up to 100 times faster because only adjacent processors interact, and we effectively leverage GPU acceleration in supercomputers,” noted study co-author Vikram Gavini, a U-M professor of mechanical engineering and materials science and engineering.
“We can now simulate glass and amorphous materials, boundaries between different crystals, as well as crystal defects that could facilitate quantum computing bits.”
The research is sponsored by the U.S. Department of Energy and utilized computing resources at the University of Texas, Lawrence Berkeley National Laboratory, and Oak Ridge National Laboratory.
“`