“`html
It is often said that an image can convey a thousand expressions.
Yet, millions of images are required to grasp the complex chemistry of an enzyme that assists in decomposing sulfur, typically present in fruits, vegetables, alcoholic beverages, and gasoline, into the odorless gas most recognized for its unique scent.
Many individuals have experienced — or more accurately, smelled — the effects when a protein enzyme known as sulfite reductase performs its function. This enzyme facilitates the chemical conversion of sulfite to hydrogen sulfide. Hydrogen sulfide is characterized by a rotten egg aroma that may arise when organic substances decompose and is frequently linked to wastewater treatment plants and landfills.

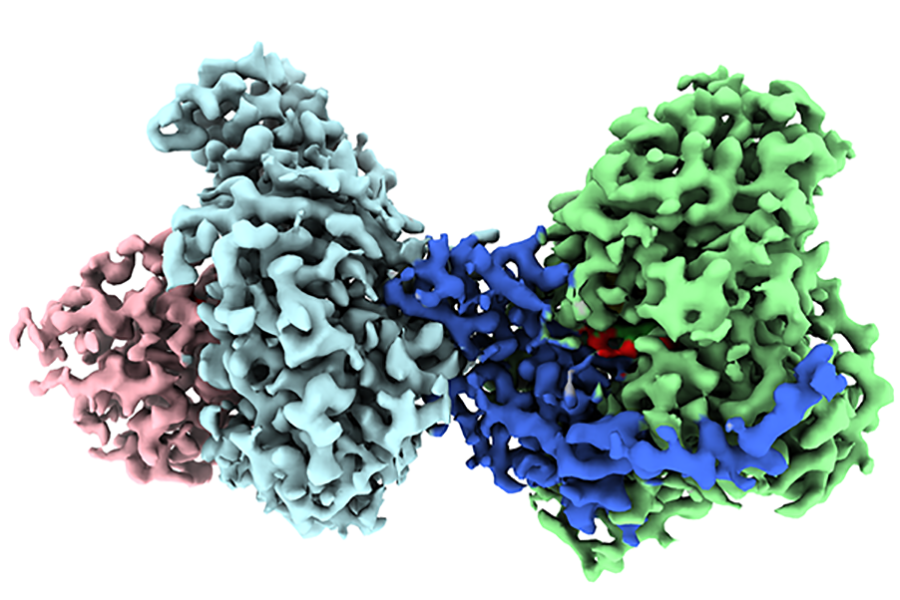
However, researchers had not succeeded in capturing a visual representation of the enzyme’s structure until this point, which has hindered their comprehensive understanding of its functionality. Elizabeth Stroupe, a Professor of Biological Science at Florida State University, along with her former doctoral student Behrouz Ghazi Esfahani, have resolved this issue and presented their findings in the journal Nature Communications.
“Artificial intelligence has improved in forecasting protein structures, but ultimately, it’s not the same as empirical data,” Stroupe remarked. “This provides us with the foundational knowledge essential for a deeper comprehension of this type of structure.”
HOW DID THE RESEARCHERS OVERCOME THIS CHALLENGE?
Stroupe and Ghazi Esfahani employed a sophisticated technique known as cryo-electron microscopy to examine the three-dimensional architecture of this enzyme. Cryo-electron microscopy permits scientists to continuously capture images of chemical processes, supplying them with the data required to visualize the structure.
To an untrained observer, protein molecules appear as intricate arrays of chemicals, yet this clear representation of the three-dimensional configuration enables scientists to discern the precise arrangement of atoms and the mechanisms of electron transfer.
“I envision it as an octopus holding four yo-yos due to the molecule’s unique flexibility,” Stroupe explained.
WHY IS THIS SIGNIFICANT?
This investigation, backed by the National Science Foundation, is vital for researchers seeking to learn how to regulate or manipulate chemical reactions—an approach frequently utilized by pharmaceutical companies or industries when developing products containing these substances.
“There are also ecological consequences,” Ghazi Esfahani noted. “Certain bacteria utilize sulfur as an energy source similarly to how humans or other organisms use oxygen. This enables us to comprehend how some of these bacteria thrive in anaerobic environments.”
WHAT ARE THE FUTURE STEPS?
This research marks a significant milestone in enhancing our understanding of the workings of sulfite reductase; however, there remain unresolved inquiries regarding its operation within a larger protein assembly and how analogous enzymes in other organisms, such as the pathogen responsible for tuberculosis, which relies on sulfur to survive within a human host, function. Stroupe’s laboratory is continuing to address this issue alongside other structural inquiries related to the sulfur metabolism process.
The article Cryo-em freezes the funk: How FSU scientists visualized a pungent protein first appeared on Florida State University News.
“`