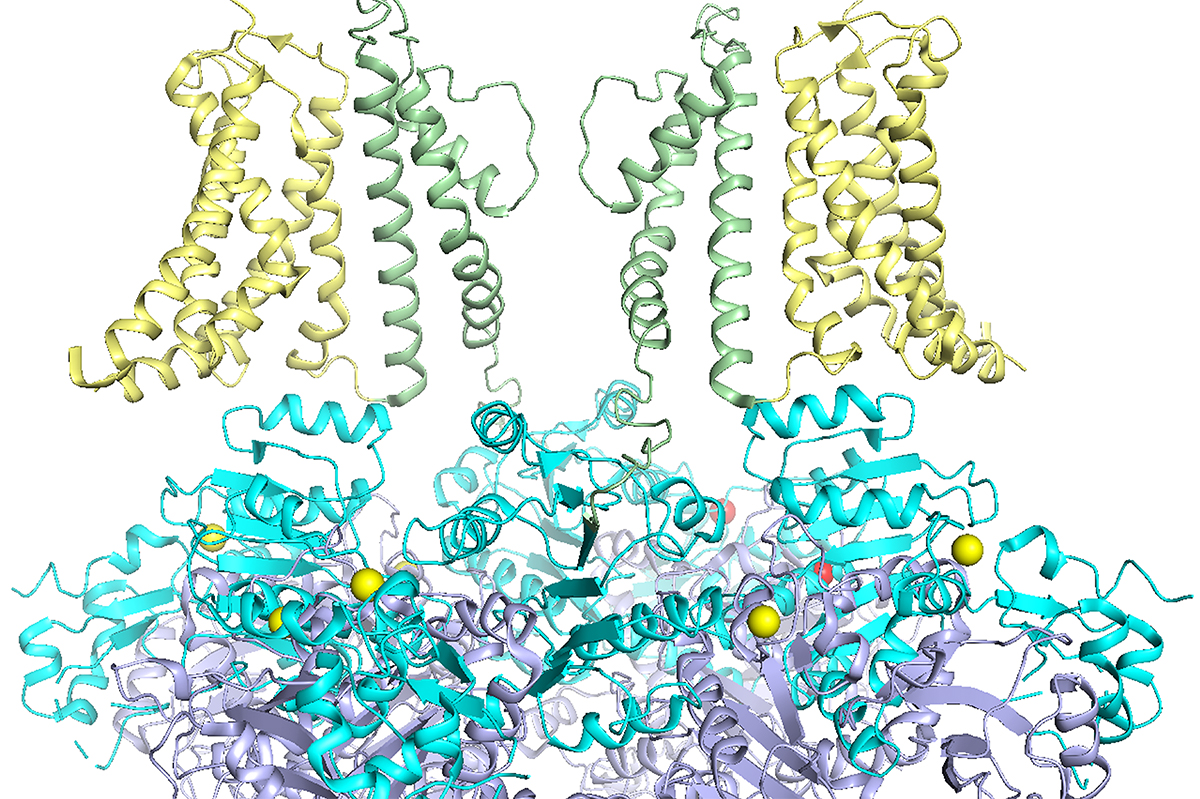
Ion channels play a crucial role in nearly all bodily functions, from maintaining regular heart rhythms to facilitating muscle movement and enabling internal communication. When any disruptions occur within these ion channels, it can lead to severe consequences for the organism.
Jianmin Cui, a faculty member specializing in biomedical engineering at the McKelvey School of Engineering, Washington University in St. Louis, aims to create tailor-made medicine for channelopathies linked to the KCNMA1 BK potassium ion channel, backed by a $3 million, five-year grant from the National Institute of Neurological Disorders and Stroke, part of the National Institutes of Health (NIH). Teaming up with him are Huanghe Yang, an associate professor of biochemistry, neurobiology, and medicine at Duke University, who possesses master’s and doctorate degrees in biomedical engineering from WashU, along with Xiaoqin Zou, a Curators’ Distinguished Professor at the University of Missouri.
Before 2005, scientists recognized that genetic alterations in potassium ion channels resulted in epilepsy and cardiac irregularities like Long QT syndrome. In that year, groundbreaking research revealed that mutations in a gene associated with the BK potassium ion channel could lead to various serious neurodevelopmental disorders, cognitive deficits, and movement disorders. These conditions exhibit distinct manifestations in almost every patient, posing challenges for caregivers and healthcare professionals alike.
Since the discovery of the first channelopathy in KCNMA1, over 40 new variants linked to neurological disorders have been documented. In the last year alone, nearly 80 additional variants have been identified. However, the mutations can occur at various points within the ion channel’s structure, resulting in an extensive array of symptoms, ranging from intellectual and developmental challenges to ataxia and dystonia.
“Some of these mutations enhance channel activity,” Cui noted. “These alterations can improve the channel’s efficiency under certain conditions. Conversely, some mutations result in a loss of function, which displays different symptoms but can also overlap. We are still unsure why an increase or decrease in function would lead to varying symptoms and affect the cells and brain in diverse manners.”
Utilizing the federal funding, Cui and his team intend to explore how these mutations influence the molecular mechanisms governing BK channel activation to ascertain whether the mutation impacts voltage-dependent activation, calcium-dependent activation, or some other intrinsic activation of the channel.
“We aim to uncover these details to devise strategies tailored for different patients,” Cui explained. “For instance, if this channel is impacted in its intrinsic opening to cause specific diseases, we will attempt a drug screening approach that directly targets that intrinsic opening, with the hope of effectively alleviating symptoms for that particular class of patients without compromising the overall channel function.”
Employing electrophysiology, Cui’s lab will investigate the molecular dynamics of the channel and how the mutations alter channel functionality leading to disease or disorder. The lab will also identify the mutation’s position within the channel, which will assist in accurately targeting potential drug development. Zou’s computational lab at the University of Missouri will conduct docking studies, placing thousands of compounds onto the specified locations of the ion channel identified by Cui’s lab to see if any will bind there and potentially modify channel function and its underlying molecular mechanisms. Yang’s lab at Duke will develop mouse models for the channel mutations to evaluate the efficacy of these compounds on symptoms.
“Currently, there is no specific medication that effectively treats this condition,” Cui remarked. “There are treatments for some symptoms, such as anti-seizure medications, but our goal is to discover a drug that targets the specific mutations to treat patients more efficiently without requiring high doses, which can often lead to additional complications.”
Cui and his team draw inspiration from the families and caregivers of individuals afflicted by these debilitating conditions.
“Although these disorders are rare, for those impacted, they can be incredibly detrimental, severely disrupting both the lives of patients and their families,” he stated. “Finding a cure is of utmost importance. There may be a genetic basis for this, and there may be pharmacological options that could assist. It represents progress in the right direction.”
Originally published on the McKelvey Engineering website
The post Genetic mutations in potassium ion channel target of new drug development appeared first on The Source.