Tuberculosis, the planet’s most lethal infectious illness, is believed to infect approximately 10 million individuals each year and claims over 1 million lives annually. Once the pathogens establish themselves in the lungs, the dense cell wall of the bacteria aids in evading the immune system of the host.
A significant portion of that cell wall consists of intricate sugar molecules referred to as glycans, yet the precise mechanisms by which these glycans safeguard the bacteria are not well comprehended. One contributing factor is the lack of straightforward methods to label them within cells.
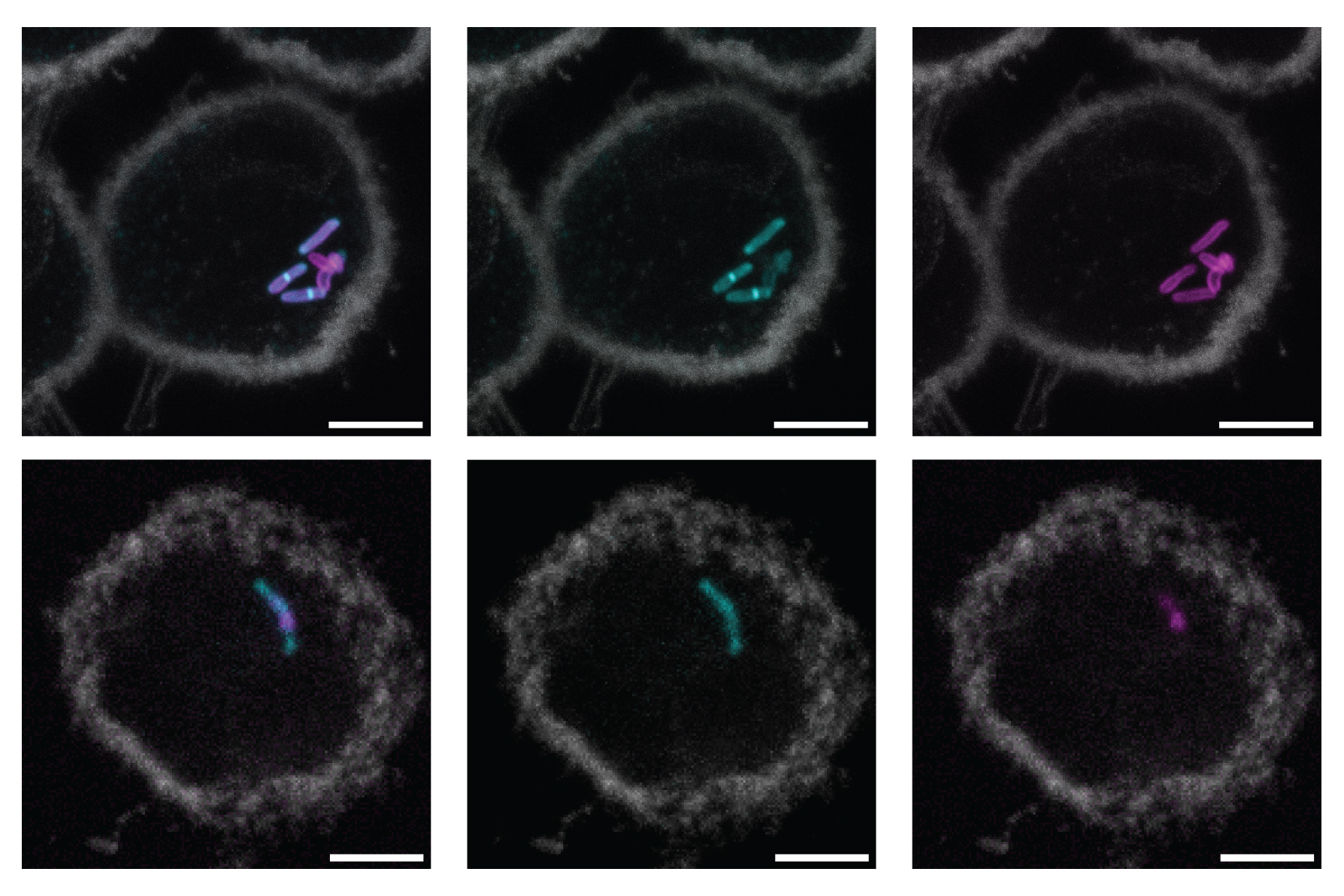
Researchers from MIT have now tackled this challenge, showcasing their ability to label a glycan known as ManLAM using an organic compound that reacts with specific sulfur-containing sugars. These sugars are present in only three bacterial species, the most infamous and widespread of which is Mycobacterium tuberculosis, the pathogen responsible for TB.
Upon labeling the glycan, the scientists could observe its location within the bacterial cell wall and investigate its behavior in the initial days of tuberculosis infection of host immune cells.
The investigators now aspire to leverage this technique to create a diagnostic tool that could identify TB-related glycans, either in culture or from a urine sample, potentially providing a more affordable and quicker alternative to current diagnostic methods. While chest X-rays and molecular diagnostics offer high accuracy, they are not consistently accessible in developing countries with elevated TB rates. In such regions, TB is frequently diagnosed by culturing microbes from sputum samples; however, this approach has a high false negative rate and can pose challenges for some patients, particularly children. Moreover, this test necessitates several weeks for bacterial growth, prolonging the diagnostic process.
“There aren’t numerous reliable diagnostic options, and certain patient groups, including children, struggle with providing samples for analysis. There’s a considerable motivation to create very straightforward, rapid tests,” states Laura Kiessling, the Novartis Professor of Chemistry at MIT and the leading author of the research.
MIT graduate student Stephanie Smelyansky is the principal author of the study, which is published this week in the Proceedings of the National Academy of Sciences. Additional contributors include Chi-Wang Ma, an MIT postdoctoral fellow; Victoria Marando PhD ’23; Gregory Babunovic, a postdoc at the Harvard T.H. Chan School of Public Health; So Young Lee, an MIT graduate student; and Bryan Bryson, an associate professor of biological engineering at MIT.
Labeling glycans
Glycans are present on the surfaces of most cells, where they fulfill vital roles such as facilitating inter-cell communication. In bacteria, glycans assist in the entry of microbes into host cells and seemingly interact with the host immune system, sometimes inhibiting the immune response.
“Mycobacterium tuberculosis possesses a highly sophisticated cell envelope compared to other bacteria, and it’s a complex structure made up of diverse glycans,” remarks Smelyansky. “One aspect that is often overlooked is that these glycans can also engage with our host cells. When our immune cells recognize these glycans, instead of triggering an alarm signal, they might convey the contrary message, indicating there is no threat.”
Glycans are notoriously challenging to tag with any probes, as they lack the unique sequences or chemical activities that can be targeted, unlike proteins or DNA. Additionally, being non-genetically encoded unlike proteins, it’s not feasible for cells to be engineered genetically to produce sugars that are labeled with fluorescent markers such as green fluorescent protein.
One significant glycan in M. tuberculosis, recognized as ManLAM, contains a rare sugar called MTX, distinctive for having a thioether — a sulfur atom situated between two carbon atoms. This chemical structure provided an opportunity to use a small-molecule tag previously developed for labeling methionine, an amino acid with a comparable structure.
The researchers demonstrated that this tag, known as an oxaziridine, could be utilized to label ManLAM in M. tuberculosis. They attached the oxaziridine to a fluorescent marker and confirmed that this tag appeared in the outer layer of the cell wall in M. tuberculosis. When the label was applied to Mycobacterium smegmatis, a non-pathogenic related bacterium lacking the sugar MTX, no fluorescent signal was detected.
“This is the first method that truly enables us to selectively visualize a specific glycan,” claims Smelyansky.
Enhanced diagnostics
The researchers also indicated that after labeling ManLAM in M. tuberculosis cells, they were able to track these cells as they infected immune cells known as macrophages. Some tuberculosis experts had theorized that bacterial cells release ManLAM once they enter a host cell, and that these free glycans then interact with the host immune system. Conversely, the MIT team discovered that the glycan seems to remain integrated within the bacterial cell walls for at least the initial days of infection.
“The bacteria continue to have their cell walls attached. Thus, while some glycan may be released, the majority appears to be retained on the surface of the bacterial cells, a phenomenon that had never been demonstrated before,” Smelyansky points out.
The researchers now intend to utilize this technique to examine what occurs to the bacteria after exposure to various antibiotics or immunological stimulation of macrophages. It may also be employed to investigate in greater detail how the bacterial cell wall is constructed, and how ManLAM facilitates the entry of bacteria into macrophages and other cells.
“Possessing a method to track the bacteria is immensely beneficial, allowing visualization of processes both in cells and in animal models that were previously obscured,” Kiessling comments.
She also envisions using this approach to devise novel diagnostics for tuberculosis. Currently, there is a diagnostic being developed that employs antibodies to identify ManLAM in urine samples. However, this test is most effective in patients with highly active cases of TB, particularly those who are immunosuppressed due to HIV or other conditions.
By using their small-molecule sensor instead of antibodies, the MIT team aims to create a more sensitive test capable of detecting ManLAM in urine even at minimal concentrations.
“This is a remarkably elegant method for selectively tagging the surface of mycobacteria, enabling real-time observation of cell wall dynamics in this significant group of bacteria. Such research will guide the creation of innovative strategies for diagnosing, preventing, and treating mycobacterial diseases, particularly tuberculosis, which continues to pose a global health challenge,” comments Todd Lowary, a distinguished research fellow at the Institute of Biological Chemistry, Academia Sinica, Taipei, Taiwan, who was not involved in the study.
The research was supported by the National Institute of Allergy and Infectious Disease, the National Institutes of Health, the National Science Foundation, and the Croucher Fellowship.