Health
Halt the hemorrhage
Terence Blue has dedicated his existence to managing hemophilia. A novel gene therapy presents respite from persistent anxiety and daily injections — ‘I am genuinely recuperating quicker than I ever have.’
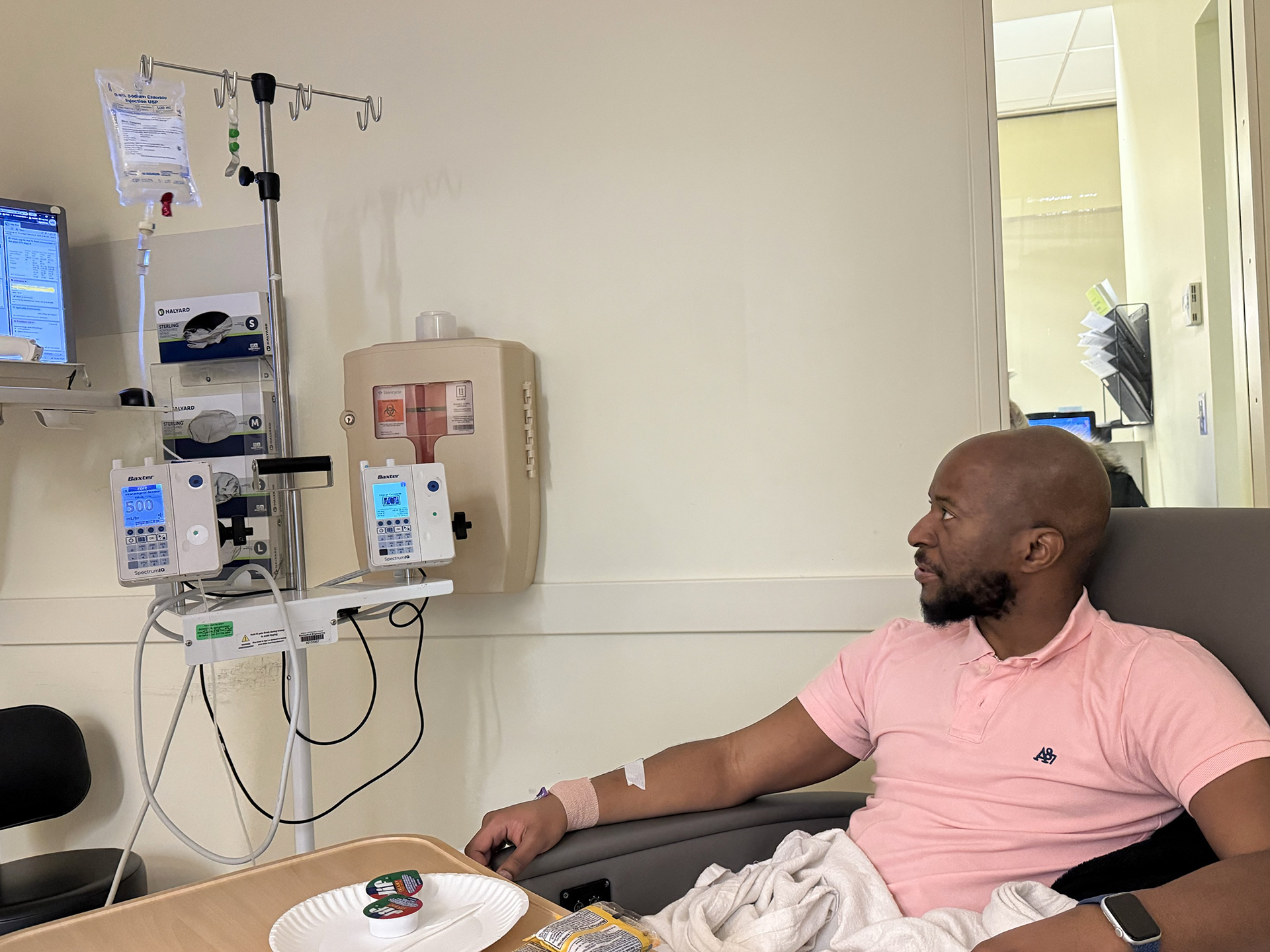
In early February, Terence Blue became the inaugural patient in New England to undergo a new gene therapy for hemophilia B, at Harvard-associated Brigham and Women’s Hospital.
The moment Terence Blue recognized his uniqueness was during a kindergarten kickball match.
The opposing team caught his kick and hurled the hefty rubber ball at him to eliminate him. Blue stumbled over the ball and struck his head on the ground, which didn’t unsettle him much. What truly affected him was the adults’ reaction, who gasped and hurried over. Fortunately, the 5-year-old had recently received clotting factor as part of his continual treatment for hemophilia. The factor functioned effectively, preventing any bleeding from the falls’ wounds and abrasions.
“I’d observed other children take spills and recall thinking, ‘What’s all the commotion?’ Then I understood I really must be exceedingly cautious about such matters,” Blue stated. “I recognized then that I needed to be vigilant.”
For the subsequent 27 years, Blue remained vigilant. Diagnosed at merely months old, for years he frequented the hospital two to three times per week for injections of the clotting factor absent from his blood. Eventually, his mother acquired the skill to administer the shots, and when he reached 8, a nurse guided him to perform the procedure independently.
Over the years, medical advancements simplified the experience of living with hemophilia. Synthetic factors eliminated the dangers of HIV, hepatitis C, and other pathogens potentially present in donated blood. New factors possess extended durability, enabling Blue to prolong the intervals between shots to two weeks. Yet, the thought of going two months without a shot remained more fantasy than fact.
“I recall being informed ‘During your lifetime, there may be a remedy,’” Blue remarked. “It always felt like a magical solution or unrealistic hope, a genie-in-the-bottle scenario. But it’s beginning to appear true. This is one step nearer. So science, let’s keep pushing forward.”
“I recall being informed ‘During your lifetime, there may be a remedy.’ It always felt like a magical solution or unrealistic hope.”
Terence Blue
In early February, Blue became the first individual in New England to receive a relatively new gene therapy for hemophilia B, at Harvard-affiliated Brigham and Women’s Hospital. Known as Hemgenix, it was created by CSL Behring and received FDA clearance in November 2022. It is part of a surge of gene and cell therapies finally emerging from the prolonged discovery process that transitions from laboratory to patients’ hospital rooms.
Market reality vs. scientists’ and patients’ aspirations
While this surge promises an expanding array of gene and cell therapies — targeting more prevalent conditions, boasting enhanced safety profiles, and improved vectors to deliver them to the body — it also implies that new treatments must confront another reality: the market. An unwavering focus on financial statements can undermine both scientists’ extensive efforts and patients’ enthusiastic hopes.
“We are witnessing a greater number of gene therapies entering clinical settings but the sector is adapting to the truth that it not only matters that gene therapies can be brought to clinics and secured FDA approval, but there are market pressures and patient acceptance that need to be factored into the situation,” stated Roger Hajjar, head of Mass General Brigham’s Gene and Cell Therapy Institute. “If the pricing is excessively high and too few patients genuinely obtain benefits from the therapies, certain sanctioned drugs in gene therapy are actually being retracted due to insufficient payers to cover them and too few patients to gain from them.”
An aspect of gene therapies’ challenges is that they provide fewer opportunities for recouping research and development expenditures. Unlike medications for chronic ailments such as diabetes, elevated cholesterol, and hypertension, which are consumed regularly over a lifetime, gene therapies are generally administered in a single dose aiming to rectify disease-causing mutations and deliver long-term advantages. This results in exorbitant costs. Blue’s treatment, for instance, has a price tag of $3.5 million, although insurance companies usually manage to negotiate lower prices, according to his physician, Nathan Connell, associate director of the Boston Bleeding Disorders Center and vice chair of the Department of Medicine at Brigham and Women’s Hospital.

Blue’s physician Nathan Connell.
Veasey Conway/Harvard Staff Photographer
This may imply limited opportunities for a market to develop and mature as patients and caregivers learn about a treatment, according to Nathan Yozwiak, head of research for Mass General Brigham’s Gene and Cell Therapy Institute. The adjustment period is often slow, he noted, and patients may not exhibit the level of enthusiasm anticipated. Drugmaker Pfizer has already withdrawn its own hemophilia B gene therapy, Beqvez, from the market less than a year following its FDA endorsement, attributing it to limited interest from patients and their healthcare providers. In 2021, Bluebird Bio removed its beta thalassemia therapy Zynteglo from the market after a disagreement with German regulators regarding its $1.8 million price tag. Even a pioneering treatment like Glybera, which addresses a rare problem in fat metabolism and ranks as the world’s debut gene therapy, was discontinued in 2017 after administering it to merely one patient over five years.
However, the enthusiasm for gene therapy’s potential to radically alter patients’ lives, perhaps indefinitely, ensuresthat progress is ongoing. Currently, the sector is gaining momentum as innovative therapies arise from the pipeline linking fundamental research to clinical settings, as per Hajjar, a trailblazer in cardiac gene therapy for heart failure. An FDA inventory of gene and cell therapies — where either healthy cells or those modified in labs are administered to patients — indicates that 44 therapies have secured approval in the United States. Two received approval in 2022, five in 2023, and 18 in 2024 for illnesses such as multiple myeloma, invasive bladder cancer, sickle cell disease — which utilized CRISPR gene-editing technology for the inaugural time — and knee cartilage defects, among others.
“Within the research domain, there’s tremendous, tremendous enthusiasm that’s mirrored in the fact that the list of diseases for which researchers are seeking a gene or cell therapy expands each year,” Yozwiak commented. “Ultimately, I believe we’re going to have several therapies that are considerably effective. Aligning that with the economic realities can sometimes be frustrating for researchers.”
‘I’m weary of needles’
Blue started discussing gene therapy with Connell two years prior after Hemgenix gained approval from the Food and Drug Administration. He noted it took several months to analyze research data independently, acclimatize to the notion of introducing foreign genes into his system, and decide to proceed. The thought that he could potentially liberate his life from the needles that have been a daily occurrence, allowing him to travel without the need for an emergency supply of factor IX — just in case — and evade the genuine social pressures that have cost him friendships became more appealing.
“I’m weary of needles. They’ve been part of my life forever,” Blue shared. “It’s a minor detail, but it wears on you.”
Once he chose to progress, it took months for the hospital to formulate its own scientific assessment, obtain internal approvals, and finalize protocols before finally, ordering the medication and administering the therapy.
The treatment capitalizes on the natural capability of viruses to target specific organs and implant viral DNA into cells’ genetic material. In this instance, bioengineers selected a virus that zeroes in on the liver—where clotting factor is produced—and substituted the virus’s DNA with a corrected version of the mutated gene responsible for hemophilia B. Once it reaches the liver, the virus integrates its payload into liver cells, kickstarting the production of clotting factor IX, which is either deficient or absent in hemophilia B, the less common of hemophilia’s two forms affecting roughly 15 percent of patients.
“Essentially, you have a bit of a Trojan horse,” Connell explained. “You aim to transport it into the liver, and you employ this mechanism to achieve that. Patients arrive at the infusion center and it’s all conducted as an outpatient procedure.”
The Future of American Higher Education
Harvard hosts some of the most advanced medical, scientific, and technological research globally. What is at stake if the government reduces funding support?
Since the genes responsible for hemophilia are located on the X chromosome, the disorder is more prevalent among males than females. Females possess two copies of the X chromosome, and even one normal gene typically allows their blood to clot properly. Males, with XY chromosomes, have only one opportunity: If their sole X chromosome carries the mutation, they develop hemophilia. The U.S. Centers for Disease Control and Prevention notes that precise statistics for individuals with hemophilia are unavailable, but a recent survey indicated approximately 33,000 American males are living with the condition.
The management of hemophilia has made significant strides, largely supported by the standardization of prophylactic injections of clotting factor for severe cases like Blue’s over the past few decades. Life expectancy was below 30 before modern hemophilia management took effect but today approaches that of the average male population, according to a recent study conducted by Canadian researchers.
“I called him and I think he was in a meeting at work. He had no idea what to expect. I was thrilled to tell him that it’s effective.”
Nathan Connell
Despite improvements, care remains incomplete, Connell stated. Unexpected bleeds can occur frequently, are hard to predict or control, and often happen internally, affecting various body parts, including the brain. It’s not uncommon for patients to experience spontaneous bleeds and awaken with a stiff elbow, knee, or another joint, indicating that blood has accumulated inside. This situation can be alleviated with an additional dose of clotting factor, but over time the bleeds can harm the joints’ smooth, slippery cartilage, leading to pain and increasing the likelihood of further bleeding. Blue, now 33, has an ankle affected by an arthritis-like condition termed hemophilic arthropathy because of trauma originating from an injury in his youth.
“Before we relied on prophylaxis, many individuals with severe hemophilia ended up in wheelchairs or needed crutches because they experienced frequent bleeding episodes and ultimately lost their ability to walk,” Connell remarked.
After decades of managing his condition, Blue noted that the physical aspects of living with hemophilia have become routine, though never far from his thoughts. However, the social aspects remain challenging and can be discouraging. He frequently has to clarify to companions why he cannot participate in certain activities and notes that disclosing his condition has resulted in lost friendships. Nowadays, unless he is engaged in an activity where he feels his companions should be aware, he remains quiet about it.
Even with its constraints, Blue has managed to lead an active life. He earned his black belt in tae kwon do at 14—wearing extra protection during sparring—and besides his role as an IT security engineer, he indulges in bachata, a type of Latin social dance, several times weekly.
For something so innovative and potentially significant, the administration of the therapy was quite standard, if not mundane. Blue’s infusion occurred on Feb. 6 and lasted around two hours. Under the close observation of Connell and other members of his care team, Blue reported minimal side effects. After an additional four hours of monitoring, he was discharged after indicating nothing was amiss. In the weeks that ensued, he commenced steroid treatment after liver enzymes increased. On Feb. 20, he received his final injection of clotting factor IX, and by mid-March, he was gradually reducing his steroid dosage as his liver function improved. By that point, his factor IX levels, which had been below 1 percent, had escalated to 32 percent, reaching the mild hemophilia to low normal range.
“We hope it proves effective. We have evidence that it works, but until you witness it starting to yield results, there is always a slight apprehension that it might not go as planned,” Connell stated. “I contacted him, and I believe he was in a meeting at work. He stepped outside when he saw the number. He didn’t know what to anticipate. I said, ‘It’s working.’ And I was genuinely thrilled to inform him that it’s effective.”
Although doctors are cautious about labeling these therapies as “cures,” there exists the potential for effects lasting several years or even decades. Ninety-four percent — 51 of 54 — of those treated with Hemgenix during the clinical trial still do not require factor IX prophylaxis three years later, according to the drugmaker’s website. Blue, who sustained a painful cut beneath his thumbnail in March, is still adjusting to the healing journey he has embarked upon.
“I’ve encountered this situation many times before, so after I panicked momentarily, I went to treat it,” Blue recounted. “My wife was sitting there watching, and within seconds I realized it was starting to heal. This turnaround is unprecedented for me. I’m classified as ‘severe,’ and I’m accustomed to bleeding episodes lasting longer. In that moment, I thought, ‘Wow, this is reality. This is effective. I haven’t had factor in ages, but here I am actually healing quicker than I ever have in my life.’”