We function through the collaboration of numerous skeletal muscle fibers, all twitching and pulling in harmony. While certain muscles align in one direction, others create complex patterns, facilitating the movement of body parts in various ways.
Recently, researchers and engineers have turned to muscles as potential actuators for “biohybrid” robots — machines powered by soft, artificially cultivated muscle fibers. Such bio-robots might wriggle and wiggle through areas where conventional machines cannot fit. For the most part, however, scientists have only managed to produce artificial muscle that exerts force in a single direction, restricting the robot’s range of movement.
Now, engineers from MIT have created a technique to cultivate artificial muscle tissue that twitches and flexes in numerous coordinated directions. As a demonstration, they developed an artificial, muscle-driven structure that pulls both concentrically and radially, similar to how the iris in the human eye expands and contracts the pupil.
The researchers constructed the synthetic iris using a novel “stamping” technique they devised. First, they 3D-printed a small, handheld stamp etched with microscopic grooves, each as tiny as a single cell. Then they pressed this stamp into a pliable hydrogel and populated the resulting grooves with actual muscle cells. The cells proliferated along these grooves within the hydrogel, forming fibers. Once stimulated, the fibers contracted in multiple directions, conforming to the fibers’ alignment.
“With the iris design, we believe we have shown the first skeletal muscle-powered robot capable of generating force in more than one direction. This was uniquely made possible by the stamping method,” states Ritu Raman, the Eugene Bell Career Development Professor of Tissue Engineering in MIT’s Department of Mechanical Engineering.
The team asserts that the stamp can be produced using typical tabletop 3D printers and can be outfitted with various patterned microscopic grooves. The stamp has potential applications to cultivate intricate patterns of muscle — and possibly other biological tissues, like neurons and cardiac cells — that resemble and behave like their natural variations.
“We aim to create tissues that replicate the complex architecture of authentic tissues,” Raman remarks. “Achieving that requires this level of precision in your fabrication.”
She and her associates published their open-access findings Friday in the journal Biomaterials Science. Her co-authors from MIT include first author Tamara Rossy, Laura Schwendeman, Sonika Kohli, Maheera Bawa, and Pavankumar Umashankar, along with Roi Habba, Oren Tchaicheeyan, and Ayelet Lesman from Tel Aviv University in Israel.
Training environment
Raman’s lab at MIT aspires to engineer biological materials that emulate the sensing, activity, and responsiveness of authentic tissues within the body. Broadly, her group seeks to leverage these bioengineered materials in fields ranging from medicine to mechanization. For example, she is aiming to fabricate artificial tissues capable of restoring function to individuals with neuromuscular injuries. She is also investigating artificial muscles for application in soft robotics, such as muscle-driven swimmers that glide through water with fish-like agility.
Raman has previously pioneered what could be likened to gym platforms and workout routines for lab-cultivated muscle cells. She and her team designed a hydrogel “mat” that promotes muscle cells to grow and fuse into fibers without detaching. She also devised a method to “exercise” the cells by genetically altering them to twitch in reaction to light pulses. Moreover, her group has developed techniques to guide muscle cells to extend in long, parallel lines, mirroring natural, striated muscles. However, it remains a challenge for her and other groups to design artificial muscle tissue that can move in various, predictable directions.
“One fascinating aspect of natural muscle tissues is that they do not merely extend in one direction. Consider, for example, the circular musculature in our iris and around the trachea. Even within our limbs, muscle cells do not run straight but at an angle,” Raman observes. “Natural muscle exhibits multiple orientations within the tissue, yet we have struggled to replicate that in our engineered muscles.”
Muscle design
In their quest to develop multidirectional muscle tissue, the team stumbled upon an unexpectedly simple concept: stamps. Influenced partly by traditional Jell-O molds, the team aimed to design a stamp with microscopic patterns that could be embedded into a hydrogel, akin to the muscle-training mats they have previously created. The patterns imprinted on the mat could then function as a guide for muscle cells to follow and grow along.
“The concept is straightforward. But how do you create a stamp featuring dimensions as small as a single cell? And how can you imprint something that is incredibly soft? This gel is much softer than Jell-O, making it challenging to cast without it tearing easily,” Raman explains.
The team experimented with different stamp designs and ultimately discovered an approach that was surprisingly effective. The researchers crafted a small, handheld stamp using high-precision printing equipment at MIT.nano, enabling them to engrave intricate patterns of grooves, each about as wide as a single muscle cell, on the stamp’s underside. Prior to pressing the stamp into a hydrogel mat, they coated the bottom with a protein that assisted in achieving an even imprint into the gel and allowed it to release without sticking or ripping.
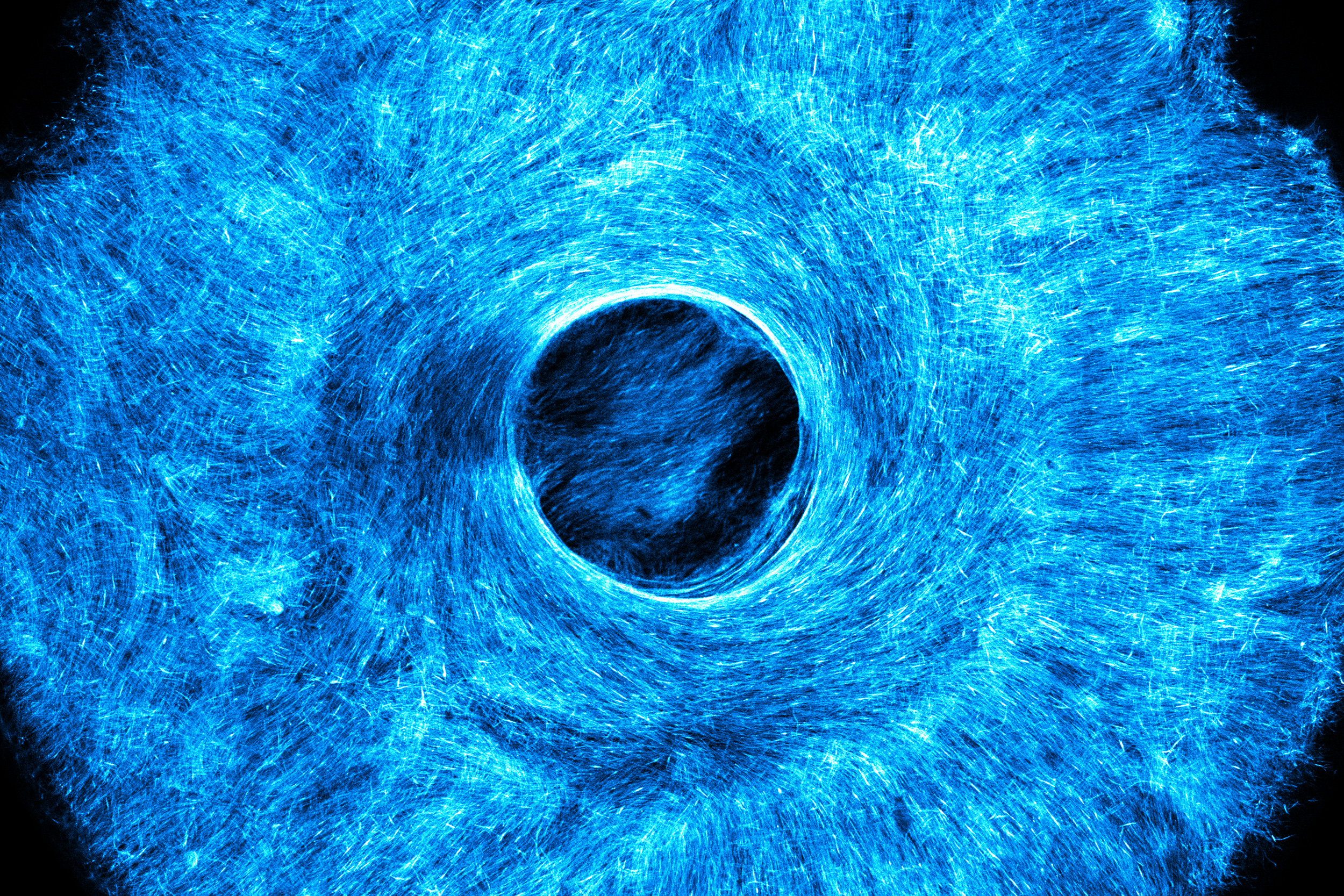
As a demonstration, the researchers printed a stamp that mirrored the microscopic structure of musculature found in the human iris. The iris consists of a ring of muscle encircling the pupil. This muscle ring comprises an inner circle of muscle fibers arranged concentrically, mirroring a circular pattern, and an outer circle of fibers that radiate outward, akin to sun rays. Together, this intricate structure serves to constrict or dilate the pupil.
Once Raman and her colleagues pressed the iris design into a hydrogel mat, they treated the mat with cells they had genetically modified to react to light. Within a day, the cells settled into the microscopic grooves and began fusing into fibers, adhering to the iris-like designs and ultimately forming a complete muscle, with an architecture and size reminiscent of a real iris.
When the team stimulated the synthetic iris with light pulses, the muscle contracted in various directions, similar to the natural iris in the human eye. Raman highlights that the team’s artificial iris is constructed from skeletal muscle cells, which facilitate voluntary movements, whereas the muscle tissue in the actual human iris consists of smooth muscle cells, a type of involuntary muscle tissue. They opted to arrange skeletal muscle cells in an iris-like pattern to showcase the capability to fabricate complex, multidirectional muscle tissue.
“In this research, we aimed to demonstrate that we can utilize this stamping technique to create a ‘robot’ that can perform tasks that previous muscle-powered robots cannot accomplish,” Raman remarks. “We chose to work with skeletal muscle cells, but there is no barrier preventing this technique from being applied to any other cell type.”
She mentions that while the team employed precision-printing methods, the stamp design can also be created using standard tabletop 3D printers. Moving forward, she and her team plan to adapt the stamping method to include other cell types, as well as investigate diverse muscle architectures and methods to activate artificial, multidirectional muscle for practical applications.
“Rather than relying on rigid actuators that are commonly found in underwater robots, if we can implement soft biological robots, we can navigate more efficiently and be significantly more energy-efficient, all while being completely biodegradable and sustainable,” Raman states. “That is what we aim to achieve.”
This research was partially funded by the U.S. Office of Naval Research, the U.S. Army Research Office, the U.S. National Science Foundation, and the U.S. National Institutes of Health.