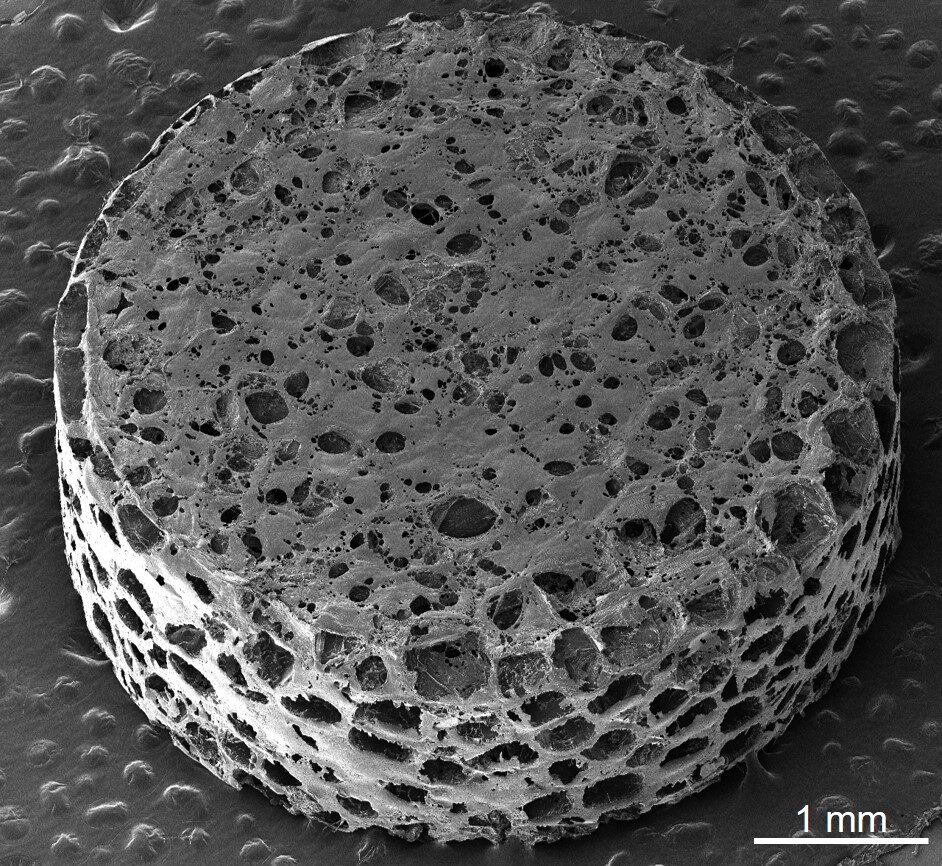
The implant draws diseased immune cells, allowing investigators to investigate and target them with a treatment based on nanoparticles
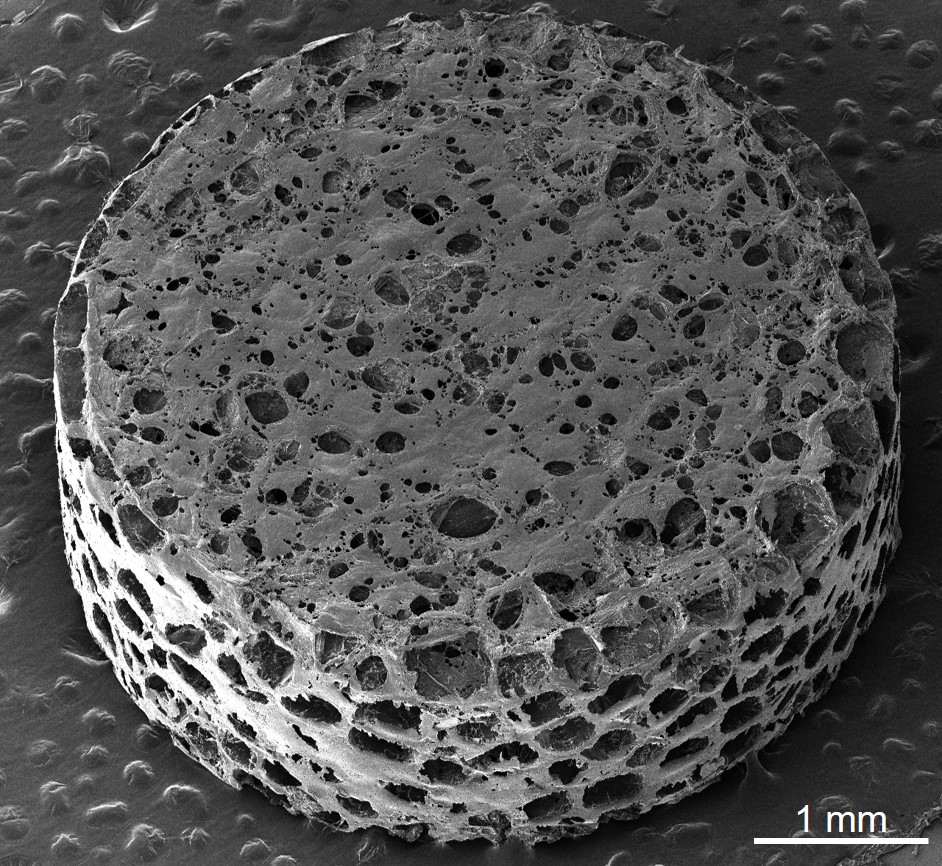
A sponge-like implant in mice guided a treatment that either slowed or halted a degenerative ailment akin to multiple sclerosis found in humans. It also offered University of Michigan researchers an initial view of how primary progressive multiple sclerosis, the most rapidly advancing form of the disease, targets the central nervous system in its early stages.
When administered at an early stage, the nanoparticle-based treatment thwarted the onset of symptoms such as paralysis in mice. If given after the initial symptoms appeared, it diminished symptom scores by fifty percent compared to those not treated.
On average, primary progressive multiple sclerosis results in significant disability within 13 years—including balance difficulties, walking impairment, and vision troubles—but symptoms can also manifest within just two years. Researchers are aware that immune cells assault the myelin sheath encasing nerves—much like insulation on an electrical wire—but pinpointing the specifics of this attack remains challenging. Since these assaults occur in the brain and spinal cord, obtaining biopsies from living patients is unfeasible.

“Currently, we have no regular means to access damaged tissue from MS patients. Some individuals donate their brains post-mortem, but at that point, the disease has advanced significantly,” stated Aaron Morris, assistant professor of biomedical engineering at U-M and co-corresponding author of the research published in the Proceedings of the National Academy of Sciences.
Lacking a thorough understanding of the disease’s mechanism, researchers have struggled to create effective treatments. At present, the sole FDA-approved medication can only slow the progression of the disease without facilitating full remission. Additionally, as it suppresses the immune response, it places patients at risk of infection.
To facilitate the development of improved therapies, the research team employed a sponge-like implant, previously utilized to diagnose relapsing MS or determine if an implanted organ is experiencing rejection. Referred to as a scaffold, it consists of a cylindrical structure made of biodegradable polyester, measuring 13 millimeters in diameter and 2 millimeters in height, filled with tiny pores for cell attachment.
Following the placement of the scaffold just beneath the skin around the shoulder blades, the team induced an MS-like autoimmune condition in half of the mice while the remaining served as healthy counterparts. Over several weeks, immune cells drawn to the foreign entity proliferated within the pores, alongside other cell types. This resulted in an easily obtainable tissue surrogate outside the central nervous system, providing insights into the dysregulated immune responses seen in MS.
The team examined the sponge tissue utilizing single-cell RNA sequencing to identify individual cell activities, which helped illuminate differences between diseased and healthy tissues. A set of proteins known as CC chemokines were notably overactive in the diseased tissues. These proteins summon other cells to combat infection, but excessive signaling leads to immune cells mistakenly attacking healthy tissues.
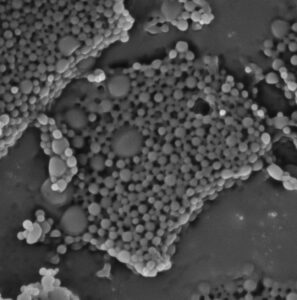
With this valuable data, the researchers developed injectable nanoparticles, measuring just 400 nanometers in diameter, which encapsulate a critical CC chemokine to interfere with the misdirected inflammation. This process effectively prevented symptoms from manifesting when mice were treated early on and reduced symptoms by over fifty percent when administered after onset. A detailed analysis of immune cells confirmed decreased disease activity in the mice treated with nanoparticles.
“The scaffold offers an unparalleled opportunity to monitor disease dynamics and explore the fundamental mechanisms, especially in the early phases. Therapies that target these initial mechanisms can hinder disease progression before substantial tissue damage occurs,” remarked Lonnie Shea, the Steven A. Goldstein Collegiate Professor of Biomedical Engineering and co-corresponding author of the research.
This study was supported by the National Institutes of Health (R00EB028840; 1R01AI155678; 1R01CA243916; R01CA272940; TDE007057), the University of Michigan Biointerfaces Institute, and the University of Michigan Precision Health Scholars.
Flow cytometry was performed at the University of Michigan Flow Cytometry Core and assays were executed utilizing the Immune Monitoring Shared Resource. The Advanced Genomics Core supplied genomic resources, and the device underwent investigation at the Michigan Center for Materials Characterization.
Shea acts as a consultant for and possesses financial interests in Cour Pharmaceuticals, which obtained licensing for the nanoparticle technology used in this investigation through the assistance of Innovation Partnerships.