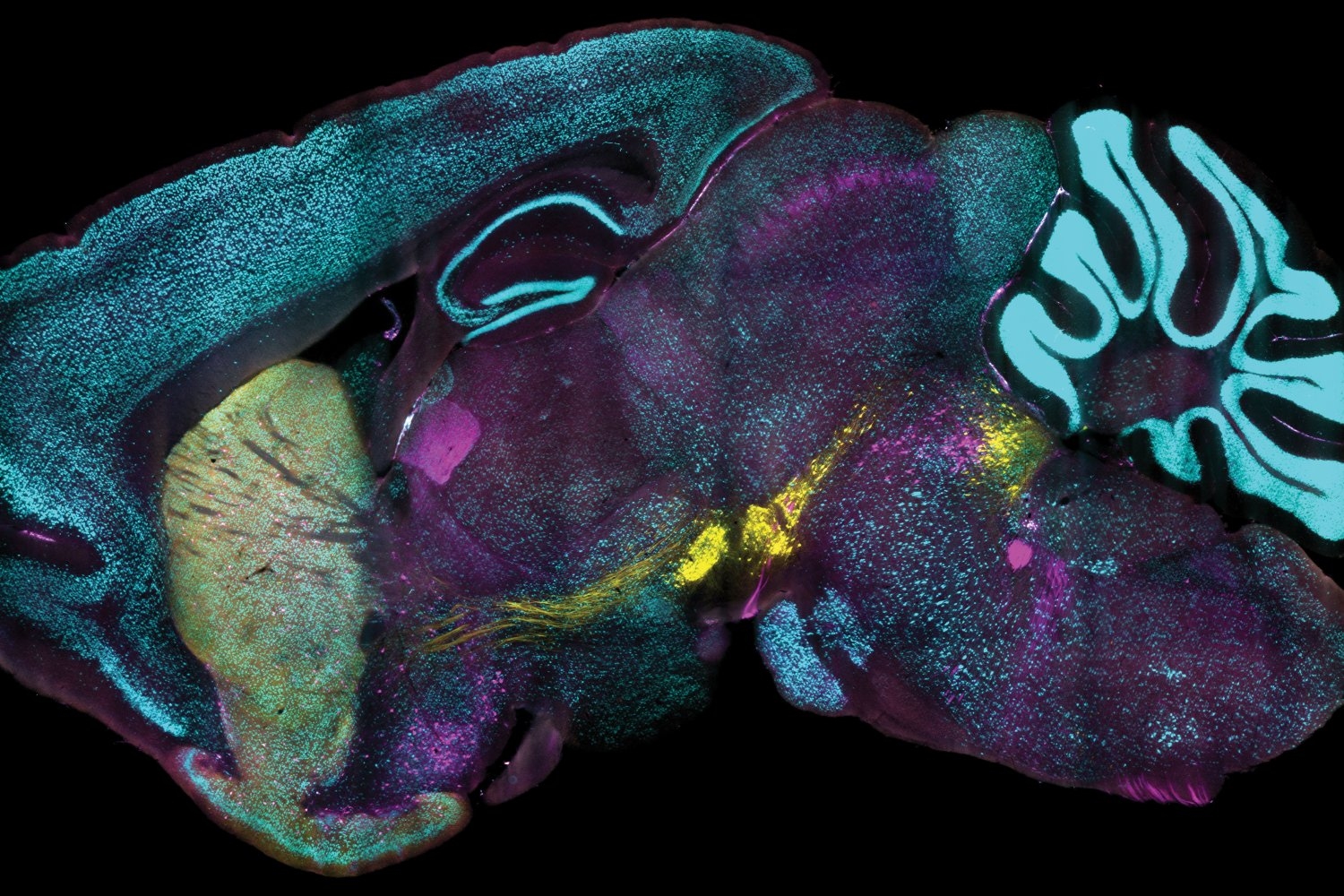
A novel technology created at MIT allows researchers to tag proteins across millions of distinct cells within fully preserved 3D tissues with unparalleled speed, consistency, and adaptability. Utilizing this technology, the research team successfully labeled extensive tissue samples within just one day. In their recent publication in Nature Biotechnology, they also illustrate that the capability to label proteins with antibodies at the individual cell level across vast tissue samples can unveil insights obscured by other commonly used labeling techniques.
Analyzing the proteins produced by cells is a fundamental aspect of research in biology, neuroscience, and related disciplines since the proteins expressed by a cell at any given moment can signify the roles the cell is attempting to fulfill or its reaction to its environment, such as illness or treatment. Although microscopy and labeling methods have progressed significantly, leading to countless discoveries, researchers have still lacked a dependable and practical means of tracking protein expression at the scale of millions of closely packed individual cells in complete, 3D intact tissues. Typically constrained to thin slices of tissue under microscope slides, scientists have thus been deprived of tools to fully appreciate cellular protein expression within the larger, interconnected systems in which it takes place.
“Traditionally, probing the molecules within cells necessitates disaggregating tissue into individual cells or sectioning it into thin slices, as the light and chemicals required for examination cannot penetrate deeply into tissues. Our laboratory has developed methods such as CLARITY and SHIELD, which allow for the examination of complete organs by rendering them transparent, but we now required a method to chemically label entire organs to obtain valuable scientific insights,” notes study senior author Kwanghun Chung, associate professor at The Picower Institute for Learning and Memory, the departments of Chemical Engineering and Brain and Cognitive Sciences, as well as the Institute for Medical Engineering and Science at MIT. “If cells within a tissue are not uniformly processed, they cannot be quantitatively compared. In traditional protein labeling, it may take weeks for these molecules to diffuse into intact organs, rendering uniform chemical processing of organ-scale tissues nearly impossible and extremely slow.”
The new methodology, termed “CuRVE,” signifies a significant breakthrough—years in development—that moves toward this objective by providing a fundamentally novel strategy for uniformly processing large and dense tissues in their entirety. In the investigation, the scientists describe how they navigated technical obstacles through an application of CuRVE dubbed “eFLASH,” and offer abundant vivid examples of the technology’s effectiveness, including how it provided fresh insights into neuroscience.
“This is a considerable advancement, particularly regarding the actual functionality of the technology,” remarks co-lead author Dae Hee Yun PhD ’24, a recent graduate of MIT who is currently a senior application engineer at LifeCanvas Technologies, a startup company established by Chung to share the innovative tools created in his lab. The paper’s other lead author is Young-Gyun Park, a former postdoctoral researcher at MIT who now serves as an assistant professor at KAIST in South Korea.
Innovative chemistry
The primary reason why large, 3D tissue specimens are challenging to label uniformly is that antibodies infiltrate tissue at a very slow rate, while simultaneously binding to their target proteins quickly. The practical consequence of this speed discrepancy is that immersing a brain in an antibody solution results in proteins being strongly labeled on the surface of the tissue, while effectively none of the antibodies reach cells and proteins located deeper within.
To enhance labeling, the team devised a method — the conceptual foundation of CuRVE — to address the speed discrepancy. The strategy involved continuously regulating the rate of antibody binding while simultaneously accelerating antibody penetration throughout the tissue. To determine how this could be achieved and to optimize the method, they developed and conducted a sophisticated computational simulation that allowed them to experiment with various settings and parameters, such as different binding rates and tissue densities and compositions.
They then set out to apply their approach to actual tissues. Their starting point was a previous technology known as “SWITCH,” which Chung’s lab created to temporarily deactivate antibody binding, allowing the antibodies to permeate the tissue before reactivating binding. Although this method proved successful, Yun explains that the team recognized there could be considerable enhancements if the speed of antibody binding could be constantly controlled; however, the chemicals employed in SWITCH were overly harsh for such continuous usage. Therefore, the team screened a library of similar compounds to identify one that could more subtly and consistently adjust antibody binding speed. They discovered that deoxycholic acid was an ideal candidate. By using this chemical, the team could not only modulate antibody binding by altering the concentration of the chemical but also by adjusting the labeling bath’s pH (or acidity).
Additionally, to expedite antibody movement through tissues, the team utilized another prior technology developed in the Chung Lab: stochastic electrotransport. This method enhances the dispersion of antibodies through tissue by applying electric fields.
Implementing this eFLASH system of accelerated dispersion with continuously adjustable binding speed led to the various labeling successes illustrated in the publication. Overall, the team reported using over 60 distinct antibodies to label proteins in cells throughout large tissue samples.
Remarkably, each of these samples was labeled within one day, an “ultra-fast” pace for entire intact organs, according to the authors. Furthermore, different preparations did not necessitate new optimization processes.
Insightful visualizations
Among the ways the team assessed eFLASH was by juxtaposing their labeling with another frequently used method: genetically modifying cells to fluoresce when the gene for a particular protein is being transcribed. The genetic approach does not require dispersing antibodies throughout the tissue; however, it may be susceptible to inconsistencies since reporting gene transcription and actual protein production are not identical. Yun added that while antibody labeling reliably and promptly indicates the presence of a target protein, the genetic method can be much less immediate and persistent, continuing to fluoresce even after the actual protein has diminished.
In the study, the team employed both labeling techniques simultaneously in samples. By visualizing the labels this way, they observed numerous instances where antibody labeling and genetic labeling varied significantly. In some areas of mouse brains, they discovered that two-thirds of the neurons expressing PV (a protein prevalent in specific inhibitory neurons) according to antibody labeling did not demonstrate any genetically-based fluorescence. In another scenario, only a minuscule percentage of cells indicating expression via the genetic approach of a protein named ChAT also displayed it through antibody labeling. In other words, there were situations where genetic labeling either significantly underreported or overreported protein expression relative to antibody labeling.
The researchers do not intend to undermine the evident importance of using genetic reporting methods; rather, they advocate that incorporating organ-wide antibody labeling, as facilitated by eFLASH, can provide a more enriched and comprehensive context to that data. “Our discovery of extensive regionalized loss of PV-immunoreactive neurons in healthy adult mice, with considerable individual variability, highlights the necessity of comprehensive and unbiased phenotyping,” the authors assert.
As Yun explains, the two distinct labeling techniques are “two different tools for the task.”
Alongside Yun, Park, and Chung, the paper’s other contributors include Jae Hun Cho, Lee Kamentsky, Nicholas Evans, Nicholas DiNapoli, Katherine Xie, Seo Woo Choi, Alexandre Albanese, Yuxuan Tian, Chang Ho Sohn, Qiangge Zhang, Minyoung Kim, Justin Swaney, Webster Guan, Juhyuk Park, Gabi Drummond, Heejin Choi, Luzdary Ruelas, and Guoping Feng.
Financial support for the study was provided by the Burroughs Wellcome Fund, the Searle Scholars Program, a Packard Award in Science and Engineering, a NARSAD Young Investigator Award, the McKnight Foundation, the Freedom Together Foundation, The Picower Institute for Learning and Memory, the NCSOFT Cultural Foundation, and the National Institutes of Health.