“`html
For years, synthetic biologists have been creating gene circuits that can be introduced into cells for uses such as transforming a stem cell into a neuron or producing a protein that may aid in the treatment of an ailment like fragile X syndrome.
These gene circuits are commonly delivered into cells by vectors such as nonvirulent viruses. Nevertheless, it has proven challenging to guarantee that these cells generate the appropriate quantity of the protein specified by the synthetic gene.
To address that challenge, MIT engineers have crafted a novel control system that enables them to set a desired protein quantity, or target point, for any gene circuit. This method also permits them to modify the target point after the circuit has been introduced.
“This is an exceptionally stable and multifunctional tool. The tool is highly modular, so there are many transgenes you could regulate with this setup,” states Katie Galloway, an assistant professor in Chemical Engineering at MIT and the lead author of the new research.
Employing this approach, the researchers demonstrated that they could prompt cells to produce consistent quantities of target proteins. In one particular application they showcased, they transformed mouse embryonic fibroblasts into motor neurons by administering elevated levels of a gene that facilitates this conversion.
MIT graduate student Sneha Kabaria is the principal author of the article, which is published today in Nature Biotechnology. Other contributors include Yunbeen Bae ’24; MIT graduate students Mary Ehmann, Brittany Lende-Dorn, Emma Peterman, and Kasey Love; Adam Beitz PhD ’25; and former MIT postdoctoral fellow Deon Ploessl.
Modulating gene expression
However, it is not always feasible to get all cells in a population to express the desired gene uniformly. One reason for this is that certain cells may acquire just one copy of the circuit, while others may obtain several. Moreover, cells exhibit natural variability in the amount of protein they produce.
This has made cell reprogramming difficult because it is challenging to ascertain that every cell in a population of skin cells, for instance, will produce an adequate amount of the required transcription factors to successfully shift into a new cell identity, such as a neuron or induced pluripotent stem cell.
In the new study, the researchers created a method to regulate gene expression levels by adjusting the spacing between the synthetic gene and its promoter. They discovered that when there was a longer DNA “spacer” between the promoter region and the gene, the expression level of the gene decreased. This additional distance, they indicated, reduces the likelihood that transcription factors bound to the promoter will effectively activate gene transcription.
To establish editable set points, the researchers included sites within the spacer that can be cleaved by an enzyme known as Cre recombinase. As sections of the spacer are removed, it aids in drawing the transcription factors closer to the gene of interest, thereby enhancing gene expression.
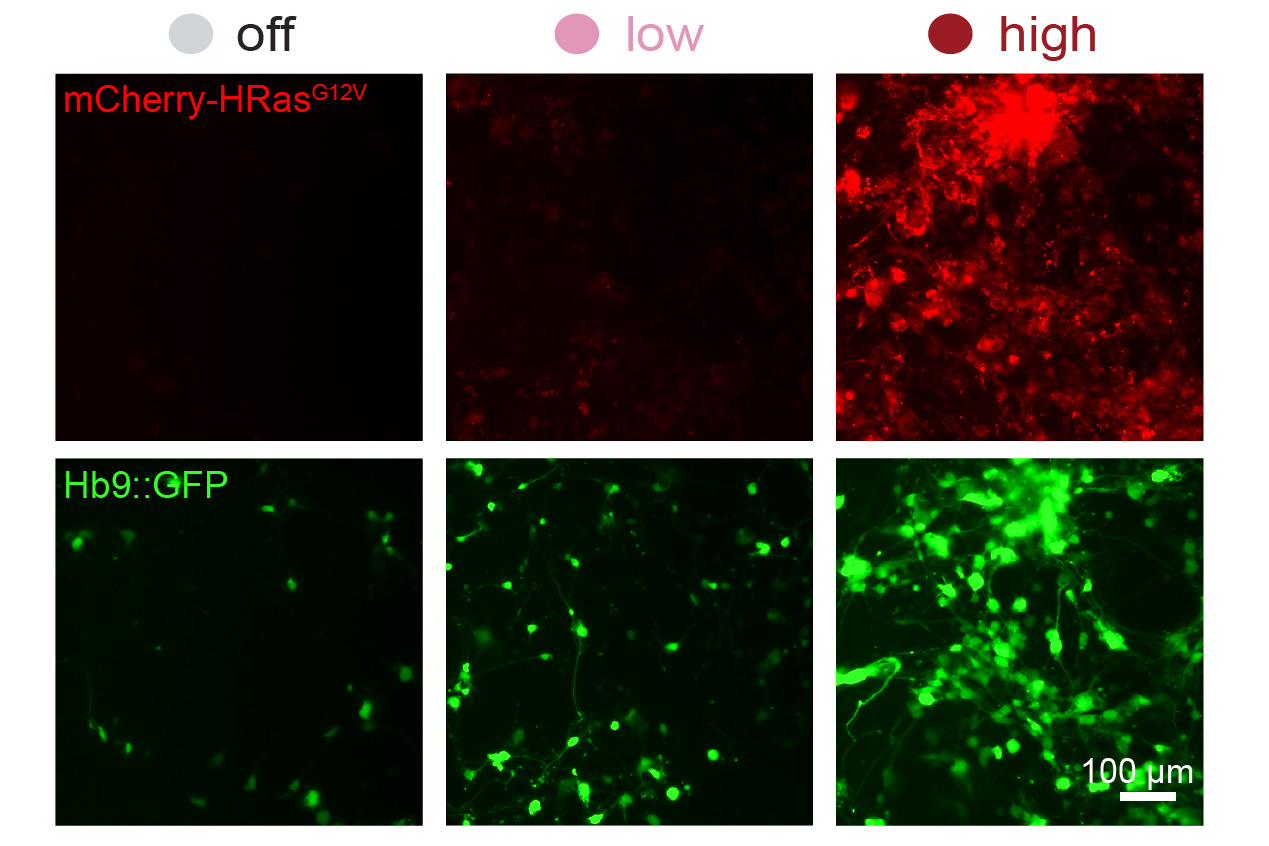
The researchers demonstrated the ability to create spacers with multiple excision sites, each targeted by different recombinases. This enabled them to develop a system referred to as DIAL, which they could use to set “high,” “medium,” “low,” and “off” target points for gene expression.
Once the DNA fragment containing the gene and its promoter is introduced into cells, recombinases can be added, allowing the target point to be modified at any time.
The researchers validated their system in mouse and human cells by introducing the gene for various fluorescent proteins and functional genes, confirming that they could achieve uniform expression across the population of cells at the target level.
“We attained consistent and stable control. This is particularly thrilling for us as a lack of uniform, stable control has been one of the obstacles limiting our capacity to develop reliable systems in synthetic biology. When excessive variables influence your system, combined with normal biological variation, it’s extremely difficult to construct stable systems,” Galloway remarks.
Reprogramming cells
To illustrate potential uses of the DIAL system, the researchers subsequently utilized it to administer varying doses of the gene HRasG12V to mouse embryonic fibroblasts. This variant of HRas has previously been demonstrated to accelerate the conversion rate of fibroblasts into neurons. The MIT team determined that in cells receiving a higher dosage of the gene, a larger proportion succeeded in transforming into neurons.
With this system, the researchers now aspire to conduct more systematic investigations of various transcription factors that can prompt cells to change into different cell types. Such investigations could elucidate how varying levels of those factors influence the success rate and whether altering the levels of transcription factors might change the type of cell generated.
In ongoing research, the researchers have shown that DIAL can be integrated with a previously developed system, known as ComMAND, that employs a feedforward loop to help prevent cells from overexpressing a therapeutic gene.
By utilizing these systems together, it may be feasible to customize gene therapies to yield specific, consistent protein levels in the target cells of individual patients, the researchers assert.
“This is something we’re enthusiastic about because both DIAL and ComMAND are highly modular, allowing for not only a well-regulated gene therapy that is somewhat general for a population but also the possibility of tailoring it for any specific individual or any particular cell type,” Galloway adds.
The research received partial funding from the National Institute of General Medical Sciences, the National Science Foundation, and the Institute for Collaborative Biotechnologies.
“`