“`html
MIT scientists have developed a novel type of fluorescent molecule that they aspire could be utilized for purposes such as producing more distinct images of tumors.
The innovative dye is derived from a borenium ion — a positively charged variant of boron that has the capability to emit light in the red to near-infrared spectrum. Until recently, these ions have been too unstable for use in imaging or other medical applications.
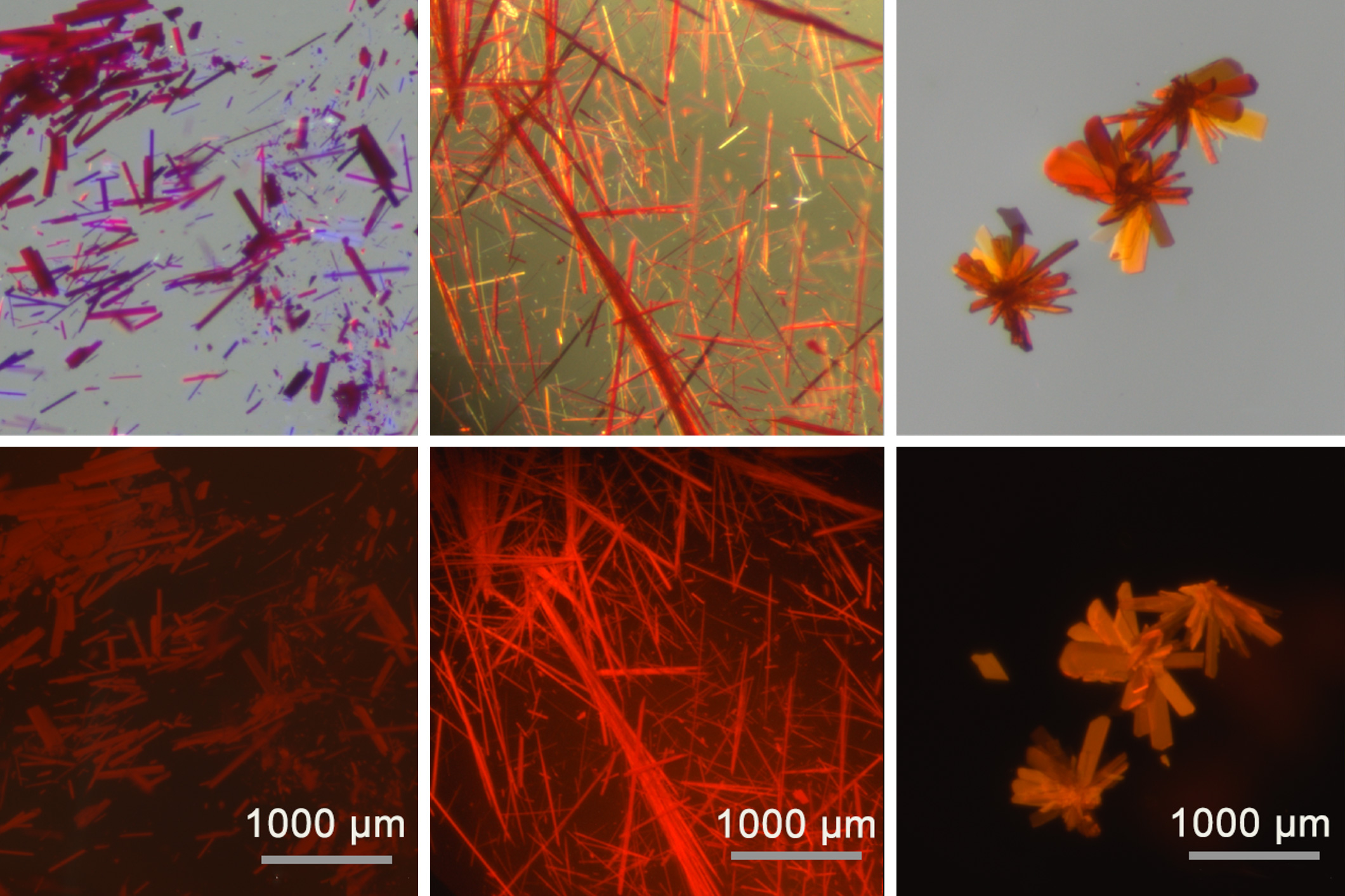
In a study published today in Nature Chemistry, the researchers demonstrated that they could stabilize borenium ions by binding them to a ligand. This method enabled them to produce borenium-infused films, powders, and crystals, all of which emit and absorb light within the red and near-infrared spectrum.
This is significant because near-IR light is more visible when imaging structures deep within tissues, potentially leading to clearer images of tumors and other structures within the body.
“One reason we focus on red to near-IR is that these types of dyes penetrate the body and tissue significantly better than light in the UV and visible range. The stability and brightness of those red dyes were the challenges we aimed to tackle in this research,” states Robert Gilliard, the Novartis Professor of Chemistry at MIT and the senior author of the paper.
MIT research scientist Chun-Lin Deng is the principal author of the article. Other contributors include Bi Youan (Eric) Tra PhD ’25, former visiting graduate student Xibao Zhang, and graduate student Chonghe Zhang.
Stabilized borenium
Most fluorescent imaging relies on dyes that emit blue or green light. These imaging agents perform well in cells, yet they are less effective in tissue because the low intensity of blue and green fluorescence generated by the body interferes with the signal. Blue and green light also scatters in tissues, restricting how deeply it can penetrate.
Imaging agents that emit red fluorescence can yield clearer images; however, most red dyes are intrinsically unstable and do not produce a bright signal due to their low quantum yields (the ratio of fluorescent photons emitted to the photons of light absorbed). For numerous red dyes, the quantum yield is only about 1 percent.
Among the molecules capable of emitting near-infrared light are borenium cations — positively charged ions featuring a boron atom attached to three additional atoms.
Upon their initial discovery in the mid-1980s, these molecules were classified as “laboratory curiosities,” Gilliard explains. They were so unstable that they had to be manipulated within a sealed container known as a glovebox to safeguard them from air exposure, which could cause them to deteriorate.
Later, chemists recognized they could enhance the stability of these ions by binding them to molecules called ligands. By working with these more stable ions, Gilliard’s lab uncovered in 2019 that they possessed some unusual attributes: Specifically, they could react to temperature variations by emitting different colors of light.
However, at that time, “there was a significant issue as they remained too reactive to be handled in open air,” Gilliard notes.
His lab began exploring new strategies to further enhance their stability using ligands known as carbodicarbenes (CDCs), which they reported in a 2022 study. Thanks to this stabilization, the compounds can now be examined and manipulated without the need for a glovebox. They are also resistant to degradation from light, unlike many prior borenium-based compounds.
In the current study, Gilliard started experimenting with the anions (negatively charged ions) that are part of the CDC-borenium compounds. Interactions between these anions and the borenium cation resulted in a phenomenon known as exciton coupling, which the researchers identified. This coupling shifted the molecules’ emission and absorption characteristics toward the infrared spectrum. These molecules also displayed a high quantum yield, enabling them to shine more brilliantly.
“We are not only in the right region, but the effectiveness of the molecules is also very commendable,” Gilliard says. “We are achieving quantum yields in the thirties for the red spectrum, which is considered significantly high for that portion of the electromagnetic spectrum.”
Potential applications
The researchers also demonstrated that they could transform their borenium-containing compounds into various states, including solid crystals, films, powders, and colloidal suspensions.
For biomedical imaging, Gilliard envisions these borenium-based materials could be encapsulated within polymers, allowing for injection into the body as an imaging dye. As an initial step, his lab aims to collaborate with researchers in the chemistry department at MIT and the Broad Institute of MIT and Harvard to investigate the imaging potential of these materials inside cells.
Due to their temperature sensitivity, these materials could also function as temperature sensors, for instance, to monitor whether drugs or vaccines have been exposed to temperatures that are excessively high or low during transit.
If integrated into thin films, these molecules could also be advantageous as organic light-emitting diodes (OLEDs), especially in novel materials such as flexible screens, Gilliard adds.
“The exceptionally high quantum yields attained in the near-IR, coupled with the remarkable environmental stability, render this class of compounds exceedingly intriguing for biological applications,” explains Frieder Jaekle, a chemistry professor at Rutgers University, who was not involved in the research. “In addition to the clear utility in bioimaging, the potent and tunable near-IR emission makes these new fluorophores highly attractive as innovative materials for anticounterfeiting, sensors, switches, and advanced optoelectronic devices.”
Apart from exploring potential applications for these dyes, the researchers are currently working on extending their color emission further into the near-infrared spectrum, which they hope to achieve by incorporating additional boron atoms. However, these extra boron atoms might render the molecules less stable, prompting the researchers to also investigate new types of carbodicarbenes to aid in stabilization.
The research received funding from the Arnold and Mabel Beckman Foundation and the National Institutes of Health.
“`