“`html
Investigators at Washington University School of Medicine in St. Louis have discovered a groundbreaking method in which brown adipose tissue — a calorie-burning type of fat — can accelerate the body’s metabolic machinery, utilizing cellular energy and generating heat in ways that enhance metabolic wellness. The research, conducted on mice, uncovers new strategies to leverage brown fat in addressing metabolic disorders such as insulin insensitivity and obesity.
The research is published on September 17 in Nature.
Brown adipose tissue is recognized for its capacity to convert energy (calories) from food into thermal energy. Conversely, white adipose tissue reserves energy for future use while muscle tissue provides immediate energy to perform tasks. The heat generated by brown fat aids in maintaining body temperature in chilly conditions, and exposure to cool environments can amplify brown fat reserves. Researchers have suggested that stimulating brown fat could aid weight-loss endeavors by enhancing calorie expenditure.
“The mechanism we’ve uncovered might offer pathways to target the energy spending aspect of the weight reduction equation, potentially simplifying the body’s ability to burn more energy by assisting brown fat in generating additional heat,” stated senior author Irfan Lodhi, a professor of medicine in the Division of Endocrinology, Metabolism & Lipid Research at WashU Medicine. “Enhancing this kind of metabolic activity could facilitate weight loss or weight regulation in a manner that may be more sustainable over time compared to conventional dieting and exercise. It’s a process that fundamentally dissipates energy — elevating resting energy expenditure — which is beneficial if you aim to lose weight.”
An auxiliary heat source in brown fat
Traditionally, brown fat’s heat production has been associated with mitochondria — the energy factories in the body’s cells. Researchers have long understood that mitochondria in brown fat possess a mechanism to disengage from energy production and instead generate heat, utilizing a molecule known as uncoupling protein 1. However, they have also been aware that mice with brown fat deficient in uncoupling protein 1 can still burn energy and produce heat, indicating the presence of alternative heating elements within cells.
The recent research highlights cellular components called peroxisomes as crucial secondary heat generators in brown fat. Peroxisomes are tiny compartments in cells that play a role in metabolizing fat molecules. Upon exposure to low temperatures, peroxisomes in brown fat increase in quantity, as noted by the researchers. This increase is even more pronounced in mice whose mitochondria lack uncoupling protein 1, suggesting that peroxisomes might be capable of compensating if mitochondria lose their heat-producing function.
Lodhi and his associates revealed that peroxisomes utilize energy and generate heat through a metabolic process centered around a pivotal protein in these cellular structures known as acyl-CoA oxidase 2 (ACOX2). Mice deficient in ACOX2 in brown fat exhibited reduced cold tolerance, displaying lower body temperatures after cold exposure compared to normal mice. Additionally, compared to typical mice, their tissues did not effectively respond to the insulin hormone that regulates blood sugar, making them more susceptible to obesity when provided high-fat diets.
In contrast, genetically modified mice that produced unusually elevated levels of ACOX2 in their brown fat demonstrated increased heat generation, enhanced cold tolerance, and better insulin sensitivity and weight management when subjected to the same high-fat diet.
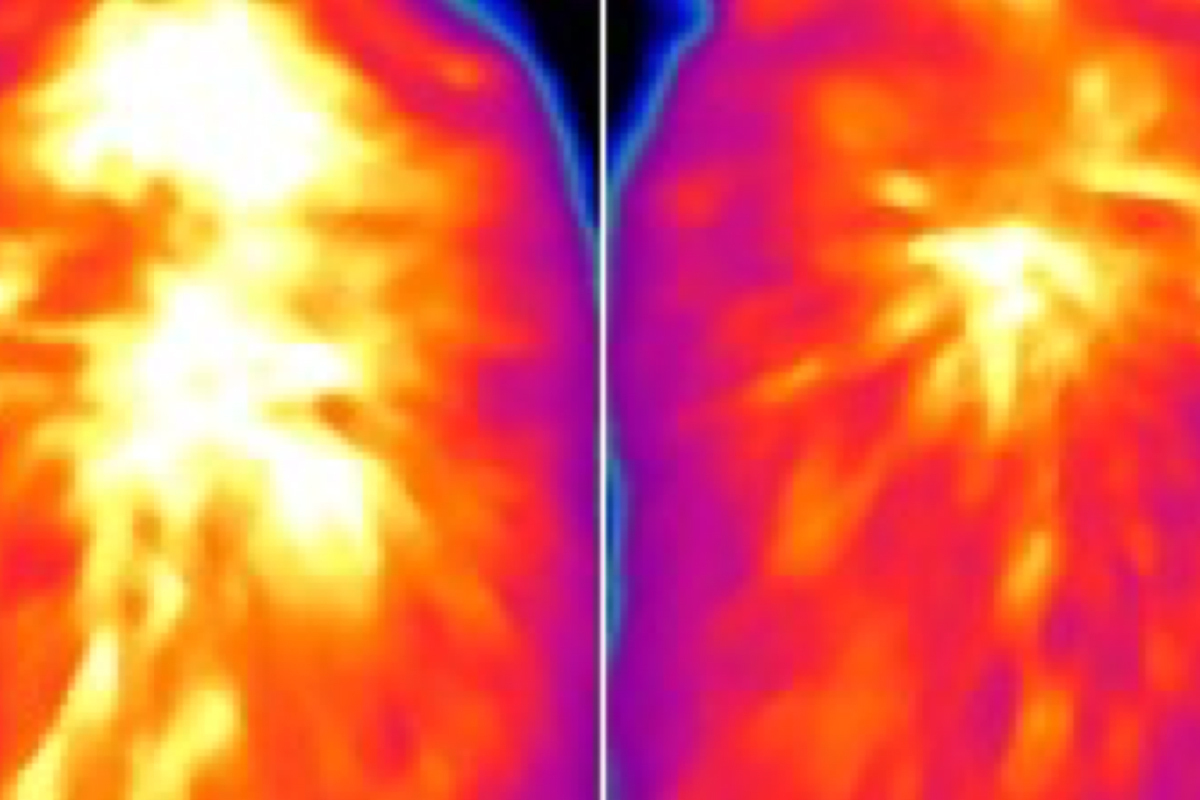
Employing a fluorescent thermal sensor they developed, the researchers found that when ACOX2 metabolized specific fatty acids, brown fat cells increased in temperature. They also utilized an infrared thermal imaging device to demonstrate that mice lacking ACOX2 generated less heat in their brown fat.
While human organisms can synthesize these fatty acids, they are also present in dairy products and human breast milk, as well as produced by certain gut microbiota. Lodhi stated this suggests the potential for a dietary approach based on these fatty acids — such as a food, probiotic, or “nutraceutical” intervention — to enhance this heat-generating pathway and its apparent beneficial effects. He and his team are also exploring potential pharmacological compounds that could directly activate ACOX2.
“Although our research focuses on mice, there is evidence indicating this pathway may be relevant for humans,” Lodhi stated. “Previous studies have shown that individuals with elevated levels of these fatty acids often have lower body mass indices. However, since correlation does not imply causation, our long-term objective is to investigate whether dietary or other therapeutic interventions that raise these fatty acids or enhance ACOX2 activity could be beneficial in stimulating this heat production pathway in peroxisomes, ultimately assisting individuals in weight loss and improving their metabolic health.”
Liu X, He A, Lu D, Hu D, Tan M, Abere A, Goodarzi P, Ahmad B, Kleiboeker B, Finck BN, Zayed M, Funai K, Brestoff JR, Javaheri A, Weisensee P, Mittendorfer B, Hsu F, Van Veldhoven PP, Razani B, Semenkovich CF, Lodhi IJ. Peroxisomal metabolism of branched fatty acids regulates energy homeostasis. Nature. Sept. 17, 2025. DOI: 10.1038/s41586-025-09517-7.
This research was funded by the National Institutes of Health (NIH), grant numbers R01DK133344, R01DK115867, R01DK132239, GM103422, T32DK007120, S10 OD032315, DK020579, and DK056341; as well as by the FP7 funded European Infrafrontier-I3 project. The views expressed are solely those of the authors and do not necessarily reflect the official positions of the NIH.
Lodhi and Liu are included on a provisional patent application submitted by Washington University regarding the targeting of ACOX2 activation as a therapy for obesity and associated metabolic disorders.
About Washington University School of Medicine
WashU Medicine is a prominent institution in academic medicine, encompassing biomedical research, patient care, and educational initiatives with 2,900 faculty members. Its National Institutes of Health (NIH) funding portfolio is the second largest among U.S. medical schools and has increased by 83% since 2016. Alongside institutional funding, WashU Medicine invests over $1 billion annually to advance basic and clinical research innovation and training. Its faculty practice consistently ranks within the top five in the nation, with more than 1,900 faculty physicians operating at 130 locations. WashU Medicine practitioners exclusively serve Barnes-Jewish and St. Louis Children’shospitals — the academic hospitals of BJC HealthCare — and provide care at BJC’s community hospitals in the region. WashU Medicine has a renowned history in MD/PhD training, recently allocated $100 million to scholarship and curriculum enhancement for its medical students, and is home to outstanding training programs in every medical specialty, along with physical therapy, occupational therapy, and audiology and communications sciences.
Originally published on the WashU Medicine website
The post New approach to ‘rev up’ brown fat burns calories, limits obesity in mice appeared first on The Source.
“`