“`html
By utilizing minute particles resembling bottlebrushes, MIT chemists have discovered a method to deliver a diverse array of chemotherapy agents directly to tumor cells.
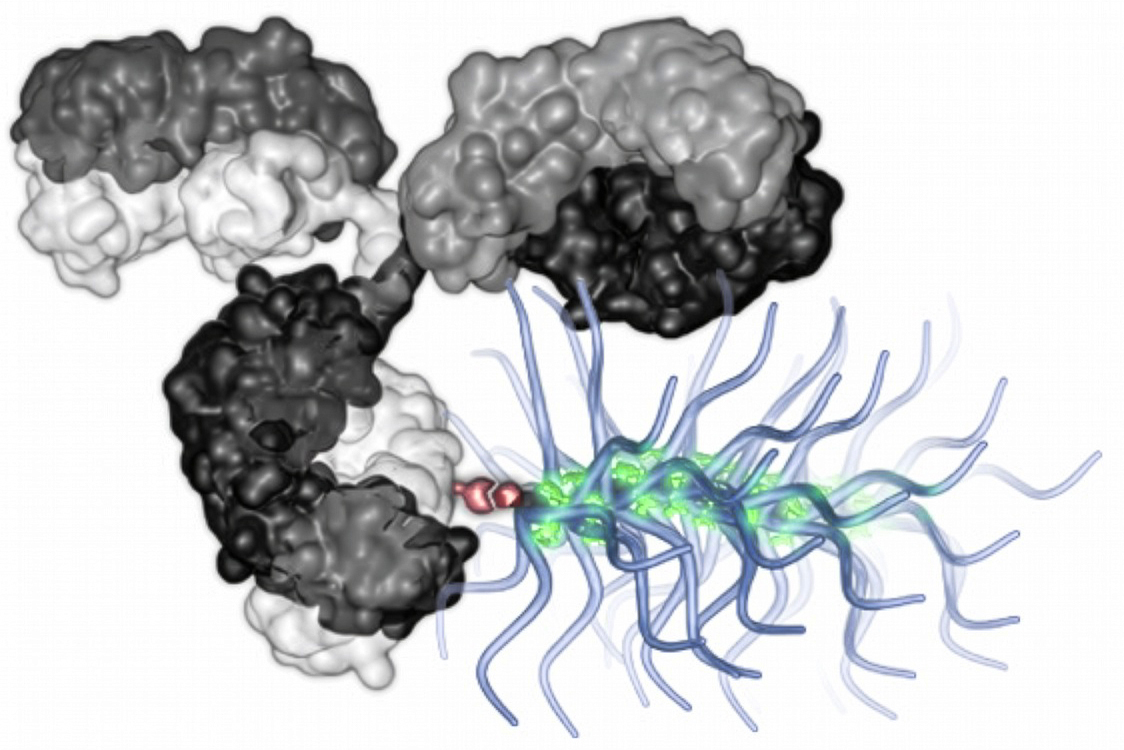
To direct them to the appropriate site, each particle is equipped with an antibody that zeroes in on a specific tumor protein. This antibody is attached to bottlebrush-like polymer chains carrying numerous drug molecules — a significantly larger cargo than what can be conveyed by any existing antibody-drug conjugates.
In murine models of breast and ovarian cancer, the researchers observed that treatment with these conjugated particles could eradicate most tumors. In the future, the particles could be adjusted to target different cancer types, by integrating various antibodies.
“We are thrilled about the prospect of unveiling a new array of payloads and payload combinations through this technology, which might ultimately yield more effective treatments for cancer patients,” states Jeremiah Johnson, the A. Thomas Geurtin Professor of Chemistry at MIT, a member of the Koch Institute for Integrative Cancer Research, and the principal author of the study.
MIT postdoctoral researcher Bin Liu is the principal author of the article, which is published today in Nature Biotechnology.
A larger drug payload
Antibody-drug conjugates (ADCs) are a promising category of cancer therapy consisting of an antibody that targets cancer attached to a chemotherapy agent. At least 15 ADCs have received approval from the FDA for various types of cancer.
This strategy enables precise targeting of a cancer drug to a tumor, thereby helping to mitigate some side effects associated with intravenous chemotherapy. Nevertheless, a limitation of currently authorized ADCs is that each antibody can link to only a handful of drug molecules. This implies they can solely be utilized with very potent drugs — typically DNA-damaging agents or compounds that disrupt cell division.
In an attempt to employ a wider variety of drugs, which are often less powerful, Johnson and his team opted to modify bottlebrush particles that they had previously developed. These particles feature a polymer backbone connected to dozens to hundreds of “prodrug” molecules — inactive drug molecules activated upon release within the organism. This design allows the particles to transport a broad spectrum of drug molecules, and they can be engineered to carry multiple drugs in tailored ratios.
Using a method known as click chemistry, the researchers demonstrated their ability to link one, two, or three of their bottlebrush polymers to a single tumor-targeting antibody, forming an antibody-bottlebrush conjugate (ABC). This configuration means that just one antibody can transport hundreds of prodrug molecules. Currently approved ADCs can carry a maximum of about eight drug molecules.
The vast quantity of payloads in the ABC particles enables the researchers to incorporate less potent cancer drugs like doxorubicin or paclitaxel, which improves the customizability of the particles and the variety of drug combinations that can be employed.
“We can utilize antibody-bottlebrush conjugates to enhance the drug loading, allowing us to use less powerful drugs,” Liu notes. “In the future, we can effortlessly copolymerize several drugs together for combination therapy.”
The prodrug molecules are linked to the polymer backbone via cleavable linkers. After the particles reach a tumor site, some of these linkers are broken immediately, permitting the drugs to eliminate nearby cancer cells even if they do not express the target antibody. Other particles are internalized by cells possessing the target antibody before releasing their toxic cargo.
Efficient treatment
For this investigation, the researchers produced ABC particles carrying various types of drugs: microtubule inhibitors named MMAE and paclitaxel, along with two DNA-damaging compounds, doxorubicin and SN-38. They also formulated ABC particles containing an experimental type of drug referred to as PROTAC (proteolysis-targeting chimera), which can selectively degrade disease-causing proteins within cells.
Each bottlebrush was connected to an antibody targeting either HER2, a protein frequently overexpressed in breast cancer, or MUC1, which is commonly present in ovarian, lung, and other cancers.
The researchers evaluated each of the ABCs in murine models of breast or ovarian cancer and determined that in most instances, the ABC particles were capable of annihilating the tumors. This treatment was markedly more effective than administering the same bottlebrush prodrugs through injection without being conjugated to a targeting antibody.
“We employed a very low dosage, nearly 100 times lower when compared to the traditional small-molecule drug, and the ABC still managed to achieve significantly better efficacy than the small-molecule drug alone,” Liu remarks.
These ABCs also outperformed two FDA-approved ADCs, T-DXd and TDM-1, both of which employ HER2 to target cells. T-DXd carries deruxtecan, which disrupts DNA replication, and TDM-1 carries emtansine, a microtubule inhibitor.
In future endeavors, the MIT team intends to explore delivering combinations of drugs that function via different mechanisms, potentially enhancing their overall effectiveness. Among these could be immunotherapy agents such as STING activators.
The researchers are also focused on substituting different antibodies, such as those targeting EGFR, which is broadly expressed in numerous tumors. More than 100 antibodies have been approved to treat cancer and other ailments, and theoretically, any of these could be linked to cancer drugs to create a targeted therapy.
The research received partial funding from the National Institutes of Health, the Ludwig Center at MIT, and the Koch Institute Frontier Research Program.
“`