“`html
Health
Scar-free brain implants
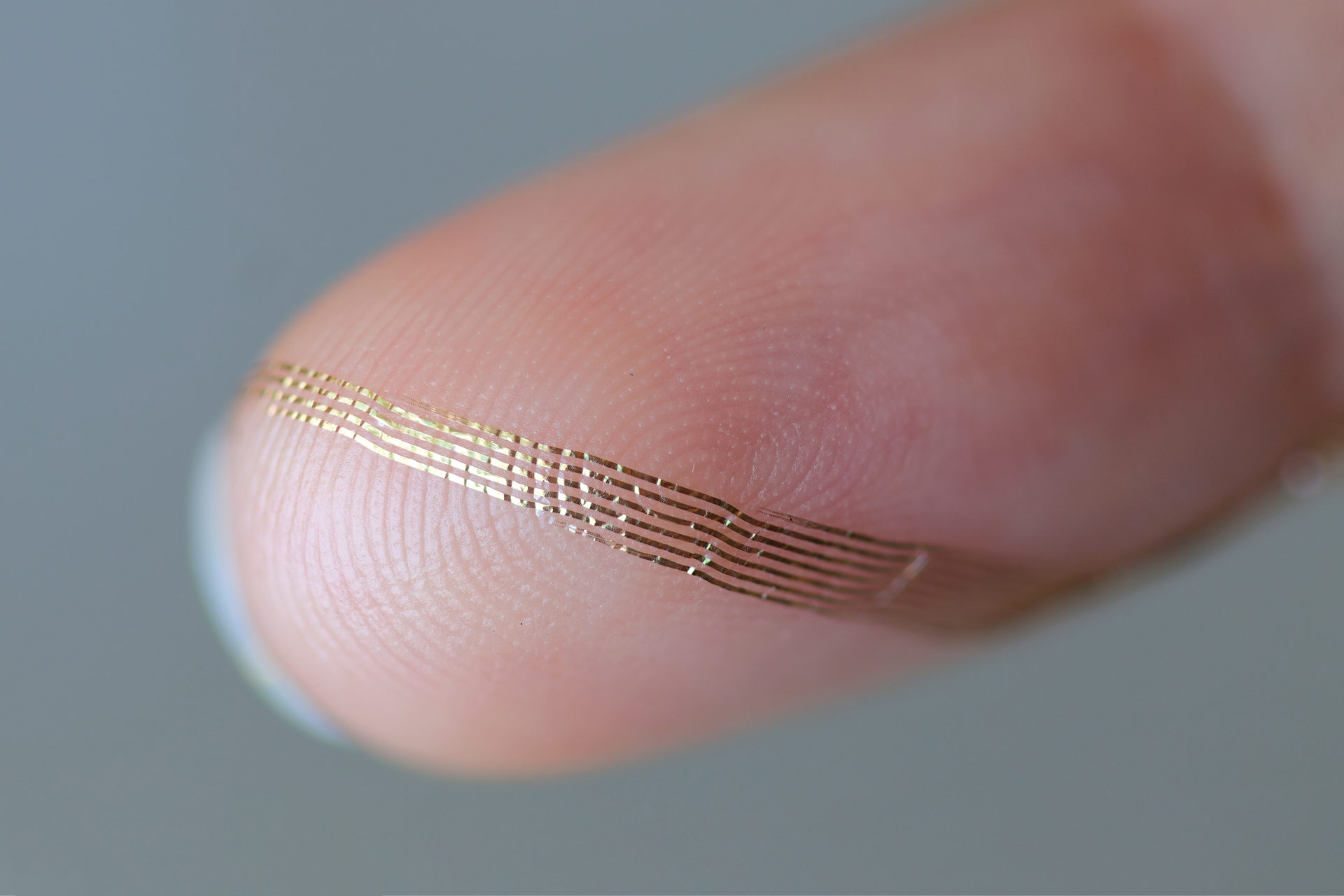
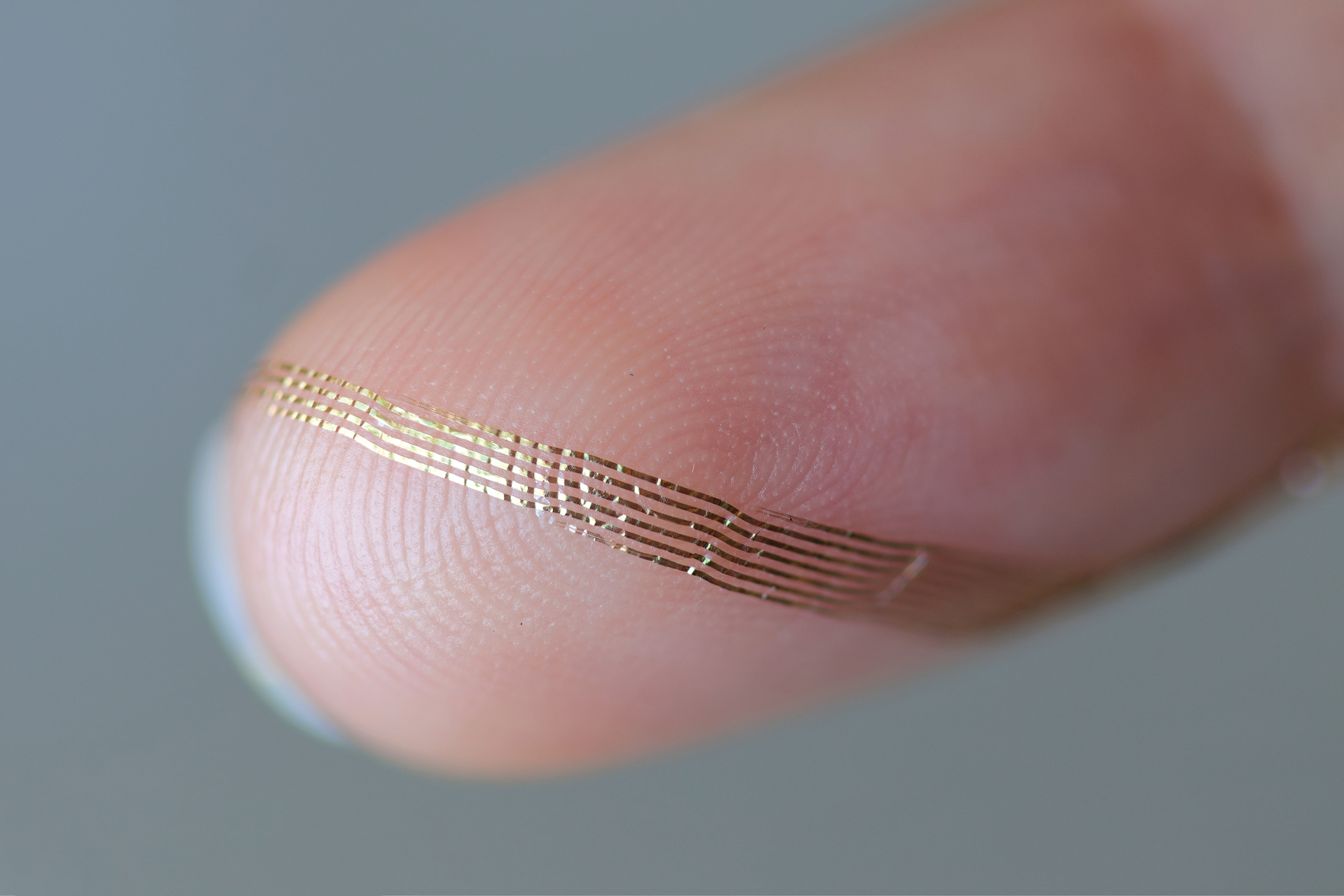
Axoft’s flexible brain implant.
Axoft Inc.
Harvard startup is crafting a gentler device to track head injuries
Traumatic brain injuries range from minor to life-threatening, yet neurologists have limited means to evaluate the harm inflicted. While assessments and external imaging are helpful, neural probes — devices that establish brain-computer interfaces — provide even greater insight. The challenge? They are constructed from inflexible materials that scar the brain.
Axoft, a startup originating from Harvard in 2021, is working on a more pliable solution, one that researchers assert can be inserted into the brain without disturbing its jelly-like texture while being robust enough to provide accurate neural data.
“With a brain-computer interface, we can ascertain very precisely what’s occurring in the patients’ brains — whether they are aware, unaware, in a vegetative state, recuperating, or if their condition is deteriorating,” stated Paul Le Floch, co-founder and CEO of Axoft, who obtained his Ph.D. in materials science from Harvard.
Clinicians have utilized neural probes for many years. Upon insertion into the brain, they gauge electrical activity with markedly greater precision than external neural imaging. Nevertheless, neural probes have historically been made from rigid materials that harm the surrounding, highly supple brain tissue — akin to razor blades in gel, mentioned Le Floch. Damage inflicted on the brain diminishes the efficiency of neural probes, as the brain responds by enveloping them with scar tissue. Encased in that tougher tissue, the probes struggle to interact freely with neighboring neurons. Additionally, inflexible devices can only remain implanted for a limited duration before they severely scar the brain. As more sensors are integrated into a neural probe — a crucial aspect for gathering extensive brain activity data — the probes become even more rigid.
Traditionally, neural probes have been made from inflexible materials, which inflict damage on the surrounding, highly pliable brain tissue — comparable to razor blades in gel.
Le Floch and his team comprehended the need for a softer substitute to existing neural probes. “The issue is: Soft materials do not perform at high levels,” he noted.
During a Ph.D. concerning material science and polymers at Harvard, Le Floch began his work as a graduate student in the laboratory of Jia Liu, an assistant professor of bioengineering at the John A. Paulson School of Engineering and Applied Sciences. Le Floch and Liu targeted a challenging issue: creating neural probes that functioned better within the brain.
Le Floch and Liu partnered with Tianyang Ye, Ph.D. ’20, a graduate student who later became a postdoctoral scholar at Harvard concentrating on nanoelectronics, as well as a fellow at the Office of Technology Development (OTD), where he focused on commercial strategies for academic innovations. Ye is currently Axoft’s chief technology officer and also a co-founder. While Le Floch developed a higher-performing, soft material that could be inserted into the brain without causing harm, Ye engineered the electronics that could relay the data for analysis.
The resulting neural probe is “extremely biocompatible, due to its diminutive size, yet also very soft,” explained Le Floch. “It inflicts less damage on tissues over time.”
Axoft’s innovative material, Fleuron, is thousands to millions of times softer and more flexible than the material commonly used in contemporary neural probes. Concurrently, Fleuron is a photoresist, suitable for the chip-fabrication process. Consequently, the probe can seamlessly accommodate over 1,000 sensors, delivering precise brain-signal information to clinicians.
“In recent decades, we’ve progressed from measuring one neuron, to 10 neurons, to hundreds of neurons — now we’re advancing to thousands,” stated Le Floch. This larger scale enables researchers to “gain more insights into the brain and devise new diagnoses and treatments.”
Axoft aims to double the number of electrodes its probe can support yearly as it continues to enhance the technology. “This will greatly boost the number of neurons Axoft’s probes can assess and stimulate,” remarked Liu, who co-founded the company and serves as a scientific advisor.
Brain implants are not essential for every individual with neurological impairment, Le Floch notes, but the company has already encountered substantial interest from neurologists who have faced challenges in monitoring the brain activity of non-responsive patients with acute and traumatic brain injuries.
“We perceive a significant need from a patient viewpoint.”
Paul Le Floch, Axoft CEO
The significance of the startup’s efforts was evident from the outset, according to Christopher Petty, OTD director of business development in physical sciences. “From our perspective, we consistently discuss this mission of transforming academically generated knowledge into a tangible impact on the world. This exemplifies that abundantly,” he remarked. “That’s the essence of our work.”
OTD safeguarded the intellectual property of the foundational discoveries, linked Axoft’s team with potential investors, and structured the startup’s license to advance the technology, while assisting its founding researchers in considering practical applications and the path from testing to commercialization.
Assisting a medical device startup in thriving, says Petty, is distinct from the protocol for a software startup. The clinical trials necessary for authorization can be lengthy and costly, yet there is also a more clearly outlined route to market. “There’s a clear set of milestones,” Petty indicated.
Since its inception, Axoft has been striving to overcome those milestones. The company has secured over $18 million in financing so far. In 2025, it conducted its first human trial at the Panama Clinic in Panama, which confirmed that the implants were safe for insertion and removal and did not introduce additional risks to the brain during the process. The team also established that the probe could distinguish between conscious and unconscious patients (due to anesthesia), the latter resembling a coma-like state. Within mere minutes, the team was able to assess brain states similarly to how a functional MRI might over several hours.
Presently, to generate further preclinical data, Axoft is collaborating with clinicians at Massachusetts General Hospital on porcine models of traumatic brain injury. Le Floch anticipates that Axoft will initiate another human study with the hospital in the coming year.
In 2027, Axoft aims for an FDA-managed clinical trial centered on individuals with traumatic brain injuries, wherein the device can track recovery and consciousness. If all proceeds smoothly, the devices could be accessible to physicians by 2028. Le Floch believes the implants could rapidly expand to hundreds of patients.
“We observe a considerable need from a patient standpoint, and an existing infrastructure in hospitals for utilizing neuromonitoring devices,” he mentioned.
This investigation received federal backing from the National Science Foundation.
“`