“`html
The thyroid, an essential endocrine gland in vertebrates, plays a crucial role in controlling metabolism and aiding growth. It is the first gland of both the nervous and endocrine systems to mature during an embryo’s development, having first emerged over 500 million years ago from a “primitive“ precursor organ in chordates known as the endostyle. Currently, utilizing lamprey as a model organism, Caltech scientists have uncovered how the evolutionary acquisition of a specific type of stem cell known as a neural crest cell facilitated the transformation of the endostyle into the thyroid.
This research is detailed in a paper published in the journal Science Advances on August 6. The study was mainly performed in the lab of Marianne Bronner, the Edward B. Lewis Professor of Biology and director of Caltech’s Beckman Institute.
Bronner’s laboratory has long concentrated on neural crest cells and their significance in vertebrate development and evolution. For instance, the team previously investigated the involvement of neural crest cells in forming bony scales that safeguard sturgeons and other primitive fish, heart tissue in zebrafish and chickens, as well as neurons of the peripheral nervous system in lamprey.
“Neural crest cells appear to encourage evolution,” Bronner states. “When Darwin initially presented the theory of evolution, he focused on the varying beak shapes of finches on the Galapagos Islands. Beaks, along with other components of the facial skeleton, are derived from neural crest cells. These cells seem capable of evolving more swiftly in evolutionary time compared to older cell types.”
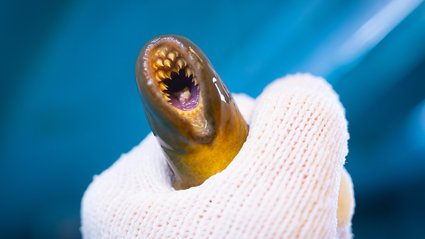
Vertebrates possess neural crest cells, whereas invertebrates do not, further intimating that these cells contribute to the evolution of intricate body forms. The Bronner lab employs lamprey, slimy parasitic eel-like fish, as a model organism because contemporary lampreys retain certain traits reminiscent of the earliest vertebrates.
The new study, directed by Senior Postdoctoral Scholar Research Associate Jan Stundl, investigates how neural crest cells aid in the development of the endostyle in lamprey. The endostyle is an evolutionary innovation of chordates (organisms within the phylum Chordata, which encompasses vertebrates), and lampreys are the sole vertebrates that maintain this organ, primarily tied to filter feeding. In lampreys, the larval endostyle, formed of two lobes in a butterfly-like configuration, metamorphoses into thyroid follicles during transformation. Stundl and the team traced the origin of five distinct cell types in the endostyle from neural crest cells, two of which develop into the thyroid follicles. Utilizing the gene-editing tool CRISPR, they genetically removed genes linked to the neural crest developmental program in lamprey embryos. These altered lampreys did not fully develop an endostyle, instead exhibiting merely a rudimentary lobe similar to the simplified endostyle of invertebrate chordates. The results indicated that neural crest cells are vital for facilitating the evolutionary shift from the chordate endostyle to the vertebrate thyroid gland.
“Mother Nature is ‘clever,’” Stundl remarks. “Rather than creating something entirely new, you can reconstruct from something already existing, like the endostyle. Neural crest cells appear to be instrumental in making this transition feasible. Without neural crest cells, we might still be filter feeders.”
The paper is titled “Acquisition of neural crest promoted thyroid evolution from chordate endostyle.” Besides Stundl and Bronner, Caltech co-authors include postdoctoral scholars Ayyappa Raja Desingu Rajan and Tatiana Solovieva; graduate student Hugo Urrutia; research associate Jana Stundlova; and former postdoctoral scholar Megan Martik now at UC Berkeley. Additional co-authors comprise Jake Leyhr, Tatjana Haitina, and Sophie Sanchez of Uppsala University; along with Zuzana Musilova of Charles University in Prague, Czech Republic. Funding was granted by the National Institutes of Health, the European Union, Alex’s Lemonade Stand Foundation, the American Heart Association, the Helen Hay Whitney Foundation, the Swedish Research Council Vetenskapsrådet, and the European Synchrotron Radiation Facility. Marianne Bronner is an affiliated faculty member with the Tianqiao and Chrissy Chen Institute for Neuroscience at Caltech.
“`